16.5 Cyclic Structures
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Structures of Monosaccharides Hemiacetals
Structures of Monosaccharides Hemiacetals • Although, the open chain structures of monosaccharides are consistent with the chemistry of carbohydrates, in reality they are oversimplifications of the true structure of carbohydrates. • It is common knowledge that aldehydes react with alcohols to form hemiacetals. In cases where a molecule is a hydroxyaldehyde such as 4- hydroxybutanal or 5-hydroxypentanal, cyclic hemiacetals result. 9:47 AM 1 Structures of Monosaccharides Hemiacetals • Aldoses often contain an aldehyde group and several hydroxyl groups as part of the same molecule; they have a greater tendency of forming cyclic hemiacetals. In fact, in aqueous solution carbohydrates exist almost exclusively in the ring-closed form At equilibrium, the linear aldehyde or ketone structure represents less than 1% of the sugar present. • Five and six-membered rings are thermodynamically more stable than their corresponding four and seven membered rings, since they are less strained. • Five- (furanoses) and six-membered cyclic hemiacetals (pyranoses) are often more stable than their open-chain forms. In particular the six-membered rings which can adopt a chair conformation are 9:47 AM 2 essentially free from all types of strains. Structures of Monosaccharides Evidence for Existence of Monosacharides as Hemiacetals What physical, chemical and spectroscopic evidence support the existence of monosaccharide sugars as cyclic hemi-acetals. (a) Two anomers of glucose capable of existing independently with different physical (melting points and specific optical rotation) and chemical properties can be obtained by recrystallization. (b) the 1H-NMR and IR-spectra of solutions of pure sugars show the presence of mixtures (anomeric hemiacetals) and absence of an aldehydic peak is a sufficient indicator that the sugars exist in some other form other than the open-chain form. -

Aldehydes Can React with Alcohols to Form Hemiacetals
340 14 . Nucleophilic substitution at C=O with loss of carbonyl oxygen You have, in fact, already met some reactions in which the carbonyl oxygen atom can be lost, but you probably didn’t notice at the time. The equilibrium between an aldehyde or ketone and its hydrate (p. 000) is one such reaction. O HO OH H2O + R1 R2 R1 R2 When the hydrate reverts to starting materials, either of its two oxygen atoms must leave: one OPh came from the water and one from the carbonyl group, so 50% of the time the oxygen atom that belonged to the carbonyl group will be lost. Usually, this is of no consequence, but it can be useful. O For example, in 1968 some chemists studying the reactions that take place inside mass spectrometers needed to label the carbonyl oxygen atom of this ketone with the isotope 18 O. 16 18 By stirring the ‘normal’ O compound with a large excess of isotopically labelled water, H 2 O, for a few hours in the presence of a drop of acid they were able to make the required labelled com- í In Chapter 13 we saw this way of pound. Without the acid catalyst, the exchange is very slow. Acid catalysis speeds the reaction up by making a reaction go faster by raising making the carbonyl group more electrophilic so that equilibrium is reached more quickly. The the energy of the starting material. We 18 also saw that the position of an equilibrium is controlled by mass action— O is in large excess. -

Educational Research Applications Abebe M, Et Al
Educational Research Applications Abebe M, et al. Educ Res Appl 5: 175. Review Article DOI: 10.29011/2575-7032.100175 Teaching Students Synthesizing Molecules Mimicking an Existing Drug against Covid-19 Moges Abebe1*, Lashan Eloise Knowles1, Bisrat Hailemeskel2 1Department of Biological and Physical Sciences, Saint Augustine University, Raleigh, NC, USA 2Department of Clinical & Administrative Pharmacy Sciences, College of Pharmacy, Howard University, NW Washington, DC, USA *Corresponding author: Moges Abebe, Department of Biological and Physical Sciences, Saint Augustine University, Raleigh, NC 27610, NC, USA Citation: Abebe M, Knowles LE, Hailemeskel B (2020) Teaching Students Synthesizing Molecules Mimicking an Existing Drug against Covid-19. Educ Res Appl 5: 175. DOI: 10.29011/2575-7032.100175 Received Date: 26 May 2020; Accepted Date: 01 June, 2020; Published Date: 06 June, 2020 Abstract End of semester organic chemistry course projects are valuable learning assessment tools while giving students a creative opportunity and sparking interest for further research investigations. The purpose of this year’s project was to teach students how to synthesize a molecule that potentially mimics an existing drug that works against the COVID-19. The available drugs chosen for the project are those that are proposed to work either by prohibiting the easy entry of the virus into respiratory tissues or those who deprive the virus’s ability to reproduce once they enter the cell. An investigative search in historical literature and the current conditions of the virus enabled students to create a unique and innovative product that requires a cumulative learned knowledge. History has shown that when a new virus becomes pandemic it takes time for researchers to create a drug, test the results, and gets approved by the Food and Drug Administration (FDA) for public availability. -

Structures of Monosaccharides Hemiacetals
Disaccharides 10:51 AM 1 Disaccharides Definition • Disaccharides are carbohydrates consisting of two monosaccharide units linked via a glycosidic bond. Non-reducing disaccharide (1,1'-Glycosidic linkage) OH HO OH O HO O OH O OH OH HO OH HO O O HO OH + HO OH Glycosidic bond OH OH HO OH HO OH 6' 6 O O Reducing end 5' 1' 4 5 HO 4' O OH 3' 2' 3 2 1 HO OH HO OH Glycone Aglycone Reducing disaccharide (1,4'-Glycosidic linkage) • These disaccharides may be reducing or non-reducing sugars depending on the regiochemistry of the glycosidic 10:51 AM linkage between the two monosaccharides. 2 Nomenclature of Disaccharides • Since disaccharides are glycosides with two monosaccharide units linked through a glycosidic bond, their nomenclature requires the formulation of priority rules to identify which of the two monosaccharides of a disaccharide provides the parent name of the disaccharide and which one will be considered the substituent. • The nomenclature of disaccharides is based on the following considerations: i. Disaccharides with a free hemiacetal group (Reducing disaccharide) ii. Disaccharides without a free hemiacetal group (Non- Reducing Disaccharide) 10:51 AM 3 Nomenclature of Reducing Disaccharides • A disaccharide in which one glycosyl unit appears to have replaced the hydrogen atom of a hydroxyl group of the other is named as a glycosylglycose. The locants of the glycosidic linkage and the anomeric descriptor(s) must be given in the full name. • The parent sugar residue in such a reducing disaccharide is chosen on the basis of the following criteria: • The parent sugar residue is the one that includes the functional group most preferred by general principles of organic nomenclature. -

Chapter 16 ! Bonds with Hidden Leaving Groups (Sections 16.1-16.6 Excluding 16.3.1 and 16.5.3 ) O Formation and Reactivity of Acetals
O Chemistry 2600 Chapter 16 ! Bonds With Hidden Leaving Groups (sections 16.1-16.6 excluding 16.3.1 and 16.5.3 ) O Formation and Reactivity of Acetals • In Chapters 7 and 15 we saw how carbonyl compounds undergo addition reactions with nucleophiles. • We also saw how some of these reactions are reversible. • For example, recall the formation of a hydrate when an aldehyde or ketone reacts with water under acidic condition: 2 O Formation and Reactivity of Acetals • This reaction is reversible and after the loss of a leaving group, the carbonyl group is reformed: • Note that the oxygen that is lost could come from the initial carbonyl oxygen atom. • We say these molecules have a ‘hidden leaving group’. • This chapter explores the chemistry of the removal or replacement of these hidden leaving groups. 3 O Formation and Reactivity of Acetals • When an aldehyde/ketone reacts with an alcohol in the presence of acid, a hemiacetal is formed. • The only difference between hemiacetal formation and hydrate formation is the nucleophile (water vs alcohol). • Hemiacetals have a hidden leaving group and in the presence of alcohol and acid, react quickly to form an acetal. • Hemiacetal (tetrahedral carbon attached to –OH and –OR) • Acetal (tetrahedral carbon attached to two –OR groups) 4 O Formation and Reactivity of Acetals • Acetal formation: 5 O Formation and Reactivity of Acetals • Acetal formation is an equilibrium process, all the steps in the sequence can run in both directions. • When an acetal is converted to a carbonyl compound, the reaction is called hydrolysis because water is being used to break (lyse) the acetal. -

20H-Carbohydrates.Pdf
Carbohydrates Carbohydrates are compounds that have the general formula CnH2nOn Because CnH2nOn can also be written Cn(H2O)n, they appear to be “hydrates of carbon” Carbohydrates are also called “sugars” or “saccharides” Carbohydrates can be either aldoses (ald is for aldehyde and ose means a carbohydrate) or ketoses (ket is for ketone) OH OH O OH CH2OH CH2OH OHC HOH2C OH OH OH OH An Aldose A Ketose (D-Glucose) (D-Fructose) Carbohydrates Due to the multiple chiral centers along a linear carbon chain for carbohydrates, Emil Fischer developed the “Fischer Projection” in order to represent these compounds Remember how to draw a Fischer projection: 1) View the linear carbon chain along the vertical axis (always place the more oxidized carbon [aldehyde in an aldose] towards the top) 2) The horizontal lines are coming out of the page toward the viewer 3) Will need to change the viewpoint for each carbon so the horizontal substituents are always pointing towards the viewer CHO OH OH H OH HO H CH2OH = OHC H OH OH OH H OH CH2OH Emil Fischer (1852-1919) Carbohydrates The aldoses are thus all related by having an aldehyde group at one end, a primary alcohol group at the other end, and the two ends connected by a series of H-C-OH groups CHO CHO CHO CHO CHO H OH H OH H OH H OH HO H CH2OH H OH H OH H OH HO H CH2OH H OH H OH HO H CH2OH H OH HO H CH2OH CH2OH Aldotriose Aldotetrose Aldopentose Aldohexose Aldohexose D-glyceraldehyde D-erythose D-ribose D-allose L-allose The D-aldoses are named according to glyceraldehyde, the D refers to the configurational -

Chapter 3 Alcohols, Phenols, and Ethers
Chapter 3 Alcohols, Phenols, and Ethers Chapter 3 Alcohols, Phenols, and Ethers Chapter Objectives: • Learn to recognize the alcohol, phenol, and ether functional groups. • Learn the IUPAC system for naming alcohols, phenols, and ethers. • Learn the important physical properties of the alcohols, phenols, and ethers. • Learn the major chemical reaction of alcohols, and learn how to predict the products of dehydration and oxidation reactions. • Learn to recognize the thiol functional group. Mr. Kevin A. Boudreaux Angelo State University CHEM 2353 Fundamentals of Organic Chemistry Organic and Biochemistry for Today (Seager & Slabaugh) www.angelo.edu/faculty/kboudrea Introduction • In this chapter, we will start looking at organic molecules that incorporate C—O bonds. • Oxygen is in Group 6A of the periodic table, and in most of its compounds, contains two single bonds and two lone pairs (or one double bond and two lone pairs), and is sp3-hybridized with a bent molecular shape: O O •The alcohol, phenol, and ether functional groups are found in a number of important naturally occurring molecules: CH3CH2OH Ethanol OH CH3CH2OCH2CH3 Diethyl ether HO Menthol Cholesterol 2 1 Chapter 3 Alcohols, Phenols, and Ethers Alcohols 3 The Hydroxy (—OH) Functional Group •The hydroxyl group (—OH) is found in the alcohol and phenol functional groups. (Note: that’s not the same as hydroxide, OH-, which is ionic.) –in alcohols, a hydroxyl group is connected to a carbon atom. –in phenols, —OH is connected to a benzene ring. (The “parent” molecule of this class is also named phenol: PhOH or C6H5OH.) • When two carbon groups are connected by single bonds to an oxygen, this is classified as the ether functional group. -

1 General Aspects of the Glycosidic Bond Formation Alexei V
j1 1 General Aspects of the Glycosidic Bond Formation Alexei V. Demchenko 1.1 Introduction Since the first attempts at the turn of the twentieth century, enormous progress has been made in the area of the chemical synthesis of O-glycosides. However, it was only in the past two decades that the scientificworldhadwitnessedadramatic improvement the methods used for chemical glycosylation. The development of new classes of glycosyl donors has not only allowed accessing novel types of glycosidic linkages but also led to the discovery of rapid and convergent strategies for expeditious oligosaccharide synthesis. This chapter summarizes major prin- ciples of the glycosidic bond formation and strategies to obtain certain classes of compounds, ranging from glycosides of uncommon sugars to complex oligosac- charide sequences. 1.2 Major Types of O-Glycosidic Linkages There are two major types of O-glycosides, which are, depending on nomen- clature, most commonly defined as a-andb-, or 1,2-cis and 1,2-trans glycosides. The 1,2-cis glycosyl residues, a-glycosides for D-glucose, D-galactose, L-fucose, D-xylose or b-glycosides for D-mannose, L-arabinose, as well as their 1,2-trans counter- parts (b-glycosides for D-glucose, D-galactose, a-glycosides for D-mannose,etc.),are equally important components in a variety of natural compounds. Representative examples of common glycosides are shown in Figure 1.1. Some other types of glycosides, in particular 2-deoxyglycosides and sialosides, can be defined neither as 1,2-cis nor as 1,2-trans derivatives, yet are important targets because of their com- mon occurrence as components of many classes of natural glycostructures. -

25.3 Cyclization of Monosaccharides
Hornback_Ch25_1085-1122 12/15/04 8:13 PM Page 1090 1090 CHAPTER 25 I CARBOHYDRATES PROBLEM 25.4 Determine the identity of each of these carbohydrates: O O CH2OH X X CH CH HO H HO H HO H HO H a) b) c) HO H HO H HO H CH2OH H OH H OH HO H CH X CH2OH O (Hint: This must be rotated first.) 25.3 Cyclization of Monosaccharides Up to this point, the structure of glucose has been shown as an aldehyde with hydroxy groups on the other carbons. However, as described in Section 18.9, aldehydes and ke- tones react with alcohols to form hemiacetals. When this reaction is intermolecular— that is, when the aldehyde group and the alcohol group are in different molecules—the equilibrium is unfavorable and the amount of hemiacetal that is present is very small. However, when the aldehyde group and the alcohol group are contained in the same molecule, as is the case in the second equation that follows, the intramolecular reaction is much more favorable (because of entropy effects; see Sections 8.13 and 18.9) and the hemiacetal is the predominant species present at equilibrium. H S O O . W Intermolecular reaction R±C±H ϩ H±.O .±R' R±C±H Equilibrium favors W reactants OS R' A hemiacetal H S O O H . Intramolecular reaction . O . OH Equilibrium favors products (6.7%) (93.3%) A cyclic hemiacetal Because glucose and the other monosaccharides contain both a carbonyl group and hy- droxy groups, they exist predominantly in the form of cyclic hemiacetals. -

Chem331 Lect 12 Carbos
Carbohydrates • Of the macromolecules that we will cover in this class, those involving carbohydrates are the most abundant in nature. • Via photosynthesis, over 100 billion metric tons of CO2 and H2O are converted into cellulose and other plant products. • The term carbohydrate is a generic one that refers primarily to carbon-containing compounds that contain hydroxyl, keto, or aldehydic functionalities. • Carbohydrates can range in sizes, from simple monosaccharides (sugars) to oligosaccharides, to polysaccharides. What Roles Do Carbohydrates Play In Vivo? Energy—Photosynthesis, (CO2+ lightàSugar + O2) Structure—cell walls and extracellular structures in plants, animals and bacteria Conjugation onto lipids, proteins—glycosylation – Molecular Recognition – Protein Folding – Solubility DNA – DNA backbone – DNA capping Carbohydrate Naming Monosaccharides—simple sugars, can’t be broken down, molecular formula (CH2O)n Oligosaccharides—a few (2-10) monosaccharides linked together (conventional names: disaccharide, etc.) Polysaccharides—polymers of simple sugars. Can have molecular weights >1x106 g/mol Monosaccharide Structure and Naming The simplest aldose and ketose are both trioses—containing 3 carbon atoms HEXOSES are the most abundant sugar in nature (think: glucose) Stereochemistry Aldoses >3 carbons and Ketoses > 4 carbons all have chiral centers. Nomenclature for sugars specifies chirality—compared to glyceraldehyde: Aldose and Ketose Tree – see your book for figure Enantiomers and Diastereomers Diastereomers have opposite conformations -

Glucose Hemi-Acetal & Acetal (Glyoside) Formation: Some
GLUCOSE HEMI-ACETAL & ACETAL (GLYOSIDE) FORMATION: SOME COMMON CONCEPTS IN CARBOHYDRATE (“SUGAR”) CHEMISTRY Carbohydrates • (carbon + hydrate) are molecules with three or more carbons and a general formula that approximates CnH2nOn Saccharides • are carbohydrates or sugars • monosaccharides have one sugar moiety • disaccharides have two sugars linked together • trisaccharides have three sugars linked together • tetrasaccharides have four sugars linked together, etc... • polysaccharides have an indeterminate number which can be hundreds of thousands or more Prefixes and Suffixes • the suffix (ending) for sugar names is: -ose • the prefix defines the number of carbons: o triose (3 carbons) o tetrose (4 carbons) o pentose (5 carbons) o hexose (6 carbons), etc… • a further prefix defines the types of carbonyl group in the sugar: o aldo- (aldehyde) or o keto- (ketone) o for example glucose (shown below) is an "aldohexose" whereas fructose is a "ketohexose" • the term pyranose means a six-membered sugar ring (hemiacetal or acetal - see below) • the term furanose means a five-membered ring • These terms are often prefixed as in "glucopyranose" which means glucose cyclized to its six-membered ring form (see below) Intro Chem Handouts Carbohydrate Chemistry Page 1 of 4 D- and L- Sugars • This is a naming convention • Using standard nomenclature numbering, determine the configuration (R or S) of the highest numbered stereogenic center ("chiral center" or "asymmetric center"): o if it has R-configuration, the sugar is a D-sugar o if it has S-configuration, the sugar is an L-sugar Glucose Hemi-Acetal Formation • The open form of D-glucose (and many other sugars) can cyclize to form hemiacetals. -
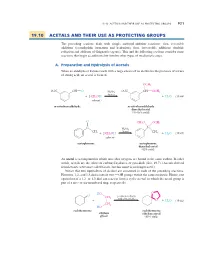
19.10 Acetals and Their Use As Protecting Groups 921
19_BRCLoudon_pgs5-0.qxd 12/9/08 11:41 AM Page 921 19.10 ACETALS AND THEIR USE AS PROTECTING GROUPS 921 19.10 ACETALS AND THEIR USE AS PROTECTING GROUPS The preceding sections dealt with simple carbonyl-addition reactions—first, reversible additions (cyanohydrin formation and hydration); then, irreversible additions (hydride reduction and addition of Grignard reagents). This and the following sections consider some reactions that begin as additions but involve other types of mechanistic steps. A. Preparation and Hydrolysis of Acetals When an aldehyde or ketone reacts with a large excess of an alcohol in the presence of a trace of strong acid, an acetal is formed. OCH3 O2N CH A O H2SO4 O2N "CH OCH3 (trace) L % % 2CH3OH % % H2O (19.44) i + (solvent) i + m-nitrobenzaldehyde m-nitrobenzaldehyde dimethyl acetal (76–85% yield) O CH O OCH S 3 3 L C L C H2SO4 L CH3 (trace) CH3 % % 2CH3OH H2O (19.45) i + (solvent) i + acetophenone acetophenone dimethyl acetal (82% yield) An acetal is a compound in which two ether oxygens are bound to the same carbon. In other words, acetals are the ethers of carbonyl hydrates, or gem-diols (Sec. 19.7). (Acetals derived from ketones were once called ketals, but this name is no longer used.) Notice that two equivalents of alcohol are consumed in each of the preceding reactions. However, 1,2- and 1,3-diols contain two OH groups within the same molecule. Hence, one equivalent of a 1,2- or 1,3-diol can react Lto form a cyclic acetal, in which the acetal group is part of a five- or six-membered ring, respectively.