Malaria Surveillance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lakshadweep Action Plan on Climate Change 2012 2012 333333333333333333333333
Lakshadweep Action Plan on Climate Change 2012 2012 333333333333333333333333 LAKSHADWEEP ACTION PLAN ON CLIMATE CHANGE (LAPCC) UNION TERRITORY OF LAKSHADWEEP i SUPPORTED BY UNDP Lakshadweep Action Plan on Climate Change 2012 LAKSHADWEEP ACTION PLAN ON CLIMATE CHANGE (LAPCC) Department of Environment and Forestry Union Territory of Lakshadweep Supported by UNDP ii Lakshadweep Action Plan on Climate Change 2012 Foreword 2012 Climate Change (LAPCC) iii Lakshadweep Action Plan on Lakshadweep Action Plan on Climate Change 2012 Acknowledgements 2012 Climate Change (LAPCC) iv Lakshadweep Action Plan on Lakshadweep Action Plan on Climate Change 2012 CONTENTS FOREWORD .......................................................................................................................................... III ACKNOWLEDGEMENTS .................................................................................................................... IV EXECUTIVE SUMMARY .................................................................................................................. XIII PART A: CLIMATE PROFILE .............................................................................................................. 1 1 LAKSHADWEEP - AN OVERVIEW ............................................................................................. 2 1.1 Development Issues and Priorities .............................................................................................................................. 3 1.2 Baseline Scenario of Lakshadweep ............................................................................................................................ -

Islands, Coral Reefs, Mangroves & Wetlands In
Report of the Task Force on ISLANDS, CORAL REEFS, MANGROVES & WETLANDS IN ENVIRONMENT & FORESTS For the Eleventh Five Year Plan 2007-2012 Government of India PLANNING COMMISSION New Delhi (March, 2007) Report of the Task Force on ISLANDS, CORAL REEFS, MANGROVES & WETLANDS IN ENVIRONMENT & FORESTS For the Eleventh Five Year Plan (2007-2012) CONTENTS Constitution order for Task Force on Islands, Corals, Mangroves and Wetlands 1-6 Chapter 1: Islands 5-24 1.1 Andaman & Nicobar Islands 5-17 1.2 Lakshwadeep Islands 18-24 Chapter 2: Coral reefs 25-50 Chapter 3: Mangroves 51-73 Chapter 4: Wetlands 73-87 Chapter 5: Recommendations 86-93 Chapter 6: References 92-103 M-13033/1/2006-E&F Planning Commission (Environment & Forests Unit) Yojana Bhavan, Sansad Marg, New Delhi, Dated 21st August, 2006 Subject: Constitution of the Task Force on Islands, Corals, Mangroves & Wetlands for the Environment & Forests Sector for the Eleventh Five-Year Plan (2007- 2012). It has been decided to set up a Task Force on Islands, corals, mangroves & wetlands for the Environment & Forests Sector for the Eleventh Five-Year Plan. The composition of the Task Force will be as under: 1. Shri J.R.B.Alfred, Director, ZSI Chairman 2. Shri Pankaj Shekhsaria, Kalpavriksh, Pune Member 3. Mr. Harry Andrews, Madras Crocodile Bank Trust , Tamil Nadu Member 4. Dr. V. Selvam, Programme Director, MSSRF, Chennai Member Terms of Reference of the Task Force will be as follows: • Review the current laws, policies, procedures and practices related to conservation and sustainable use of island, coral, mangrove and wetland ecosystems and recommend correctives. -

Agatti Island, UT of Lakshadweep
Socioeconomic Monitoring for Coastal Managers of South Asia: Field Trials and Baseline Surveys Agatti Island, UT of Lakshadweep Project completion Report: NA10NOS4630055 Project Supervisor : Vineeta Hoon Site Coordinators: Idrees Babu and Noushad Mohammed Agatti team: Amina.K, Abida.FM, Bushra M.I, Busthanudheen P.K, Hajarabeebi MC, Hassan K, Kadeeshoma C.P, Koyamon K.G, Namsir Babu.MS, Noorul Ameen T.K, Mohammed Abdul Raheem D A, Shahnas beegam.k, Shahnas.K.P, Sikandar Hussain, Zakeer Husain, C.K, March 2012 This volume contains the results of the Socioeconomic Assessment and monitoring project supported by IUCN/ NOAA Prepared by: 1. The Centre for Action Research on Environment Science and Society, Chennai 600 094 2. Lakshadweep Marine Research and Conservation Centre, Kavaratti island, U.T of Lakshadweep. Citation: Vineeta Hoon and Idrees Babu, 2012, Socioeconomic Monitoring and Assessment for Coral Reef Management at Agatti Island, UT of Lakshadweep, CARESS/ LMRCC, India Cover Photo: A reef fisherman selling his catch Photo credit: Idrees Babu 2 Table of Contents Executive Summary 7 Acknowledgements 8 Glossary of Native Terms 9 List of Acronyms 10 1. Introduction 11 1.1 Settlement History 11 1.2 Dependence on Marine Resources 13 1.3 Project Goals 15 1.4 Report Chapters 15 2. Methodology of Project Execution 17 2.1 SocMon Workshop 17 2.2 Data Collection 18 2.3 Data Validation 20 3. Site Description and Island Infrastructure 21 3.1 Site description 23 3.2. Community Infrastructure 25 4. Community Level Demographics 29 4.1 Socio cultural status 29 4.2 Land Ownership 29 4.3 Demographic characteristics 30 4.4 Household size 30 4.5. -

The Union Territories (Direct Election to the House of the People) Act, 1965 Arrangement of Sections ___
THE UNION TERRITORIES (DIRECT ELECTION TO THE HOUSE OF THE PEOPLE) ACT, 1965 _______________ ARRANGEMENT OF SECTIONS ___________ SECTIONS 1. Short title. 2. Definitions. 3. Direct election to fill the seats in the House of the People allotted to certain Union territories. 4. [Repealed.] 5. [Repealed.] 6. Provision as to sitting member. 1 THE UNION TERRITORIES (DIRECT ELECTION TO THE HOUSE OF THE PEOPLE) ACT, 1965 ACT NO. 49 OF 1965 [22nd December, 1965.] An Act to provide for direct election in certain Union territories for filling the seats allotted to them in the House of the People and for matters connected therewith. BE it enacted by Parliament in the Sixteenth Year of the Republic of India as follows:— 1. Short title.—This Act may be called the Union Territories (Direct Election to the House of the People) Act, 1965. 2. Definitions.—In this Act— (a) “parliamentary constituency” has the same meaning as in the Representation of the People Act, 1950 (43 of 1950); (b) “sitting member” means a person who immediately before the commencement of this Act is a member of the House of the People; (c) “Union territory” means any of the Union territories of the Andaman and Nicobar Islands, 1[Lakshadweep] and Dadra and Nagar Haveli. 3. Direct election to fill the seats in the House of the People allotted to certain Union territories.—At the next general election to the House of the People and thereafter, the seats allotted under section 3 of the Representation of the People Act, 1950 (43 of 1950) to the Union territories in the House of the People shall be seats to be filled by persons chosen by direct election and for that purpose each Union territory shall form one parliamentary constituency. -
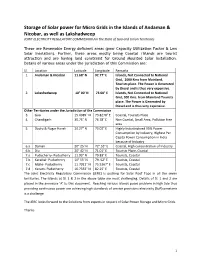
Storage of Solar Power for Micro Grids in the Islands of Andaman
Storage of Solar power for Micro Grids in the Islands of Andaman & Nicobar, as well as Lakshadweep JOINT ELECTRICITY REGULATORY COMMISSION For the State of Goa and Union Territories These are Renewable Energy deficient areas (poor Capacity Utilization Factor & Low Solar Insolation). Further, these areas mostly being Coastal /Islands are tourist attraction and are having land constraint for Ground Mounted Solar Installation. Details of various areas under the jurisdiction of this Commission are: Sl. Location Latitude Longitude Remarks 1. Andaman & Nicobar 11.68° N 92.77° E Islands, Not Connected to National Grid, 1000 Kms from Mainland, Tourists place. The Power is Generated by Diesel and is thus very expensive. 2. Lakashadweep 10° 00' N 73.00° E Islands, Not Connected to National Grid, 300 Kms. from Mainland Tourists place. The Power is Generated by Diesel and is thus very expensive. Other Territories under the Jurisdiction of the Commission 3. Goa 15.4989° N 73.8278° E Coastal, Tourists Place 4. Chandigarh 30.75° N 76.78° E Non Coastal, Small Area, Pollution free area 5. Dadra & Nagar Haveli 20.27° N 73.02° E Highly Industrialized 95% Power Consumption by Industry, Highest Per Capita Power Consumption in India because of Industry 6.a Daman 20° 25' N 72°.53° E Coastal, High concentration of Industry 6.b Diu 20° 42' N 71.01° E Tourists Place, Coastal 7.a Puducherry- Puducherry 11.93° N 79.83° E Tourists, Coastal 7.b Karaikal- Puducherry 10° 55' N 79. 52° E Tourists, Coastal 7.c Mahe- Puducherry 11.7011° N 75.5367° E Tourists, Coastal 7.d Yanam- Puducherry 16.7333° N 82.25° E Tourists, Coastal The Joint Electricity Regulatory Commission (JERC) is pushing for Solar Roof Tops in all the seven territories. -

(A) Severe Cyclonic Storm MAHA
India Meteorological Department Earth System Science Organisation (Ministry of Earth Sciences) BULLETIN NO. : 12 (ARB/04/2019) and BULLETIN NO. : 57 (ARB/03/2019) TIME OF ISSUE: 1900 HOURS IST DATED: 31.10.2019 FROM: INDIA METEOROLOGICAL DEPARTMENT (FAX NO. 24643965/24699216/24623220) TO: CONTROL ROOM, NDM, MINISTRY OF HOME AFFAIRS (FAX.NO. 23092398/23093750) CONTROL ROOM NDMA (FAX.NO. 26701729) CABINET SECRETARIAT (FAX.NO.23012284, 23018638) PS TO HON’BLE MINISTER FOR S & T AND EARTH SCIENCES (FAX NO.23316745) SECRETARY, MOES, (FAX NO. 24629777) H.Q. (INTEGRATED DEFENCE STAFF AND CDS) (FAX NO. 23005137/23005147) DIRECTOR GENERAL, DOORDARSHAN (23385843) DIRECTOR GENERAL, AIR (23421101, 23421105, 23421219) PIB MOES (FAX NO. 23389042) UNI (FAX NO. 23355841) D.G. NATIONAL DISASTER RESPONSE FORCE (NDRF) (FAX NO. 26105912, 2436 3260) DIRECTOR, PUNCTUALITY, INDIAN RAILWAYS (FAX NO. 23388503) CHIEF SECRETARY, KERALA (FAX NO. 0471-2327176) ADMINISTRATOR, LAKSHADWEEP ISLANDS (FAX NO. 0489-6263180) ADMINISTRATOR, UNION TERRITORY OF DAMAN & DIU (0260-2230775) ADMINISTRATOR, UNION TERRITORY OF DADRA NAGAR HAVELI (0260-2230775) CHIEF SECRETARY, KARNATAKA (FAX NO. 080-22258913) CHIEF SECRETARY, TAMILNADU (FAX NO.044-25672304) CHIEF SECRETARY, KERALA (FAX NO.0471-2327176) CHIEF SECRETARY, GOA (FAX NO. 0832-2415201) CHIEF SECRETARY, MAHARASHTRA (FAX NO. 022- 22028594) CHIEF SECRETARY, GUJARAT (FAX NO. 079-23250305) Sub: (A) Severe Cyclonic Storm MAHA (Pronounced as M’maha) over eastcentral Arabian Sea and adjoining Lakshadweep area: CYCLONE WARNING FOR -

Marine Fisheries Information Service
V. MARINE FISHERIES INFORMATION SERVICE SPECIAL rSSlJE ON LAKSHADWEEP 71° 72 .» CHETLAT BITRA 0 'J» KIUTAN ^KADMAT rhinakara, ^ *>•> j'/ Bangarairy,S> ,'' / K<3lP'* '.2 , ^KAVARATTI Suheli valiakara. Suheli cheriakard LAKSHADWEEP GROUP OF ISLANDS Names of inhabited islands Q\% written inronnan letters and uninhabited in ifalics 71' No, 68 1986 ANCILLARY LIVING MARINE RESOURCES OF LAKSHADWEEP K. C. George, P. A. Thomas, K. K. Appukuttan and G. Gopakumar Central Marine Fisheries Research Institute, Cochin Introduction The major agarophytes observed were Gelidiella acerosa, Gracilaria edulis, Gelidium rigidum and Gelidio- Till recently the Lakshadweep archipelago was not psis repens. Alginate resources were meagre represen investigated upon seriously from a resource point of view. In the latter half of the 19th century attempts ted by Turbinaria and Sargassum, observed in Kalpeni, have been made by some British naturalists to study the Androth and Minicoy. Among the sea weeds categoried fauna and flora of the Lakshadweep and Maldive Archi as 'others' Halimeda, Dictyota, Laurencia, Jania, Toly- pelagoes. (Alcock, 1895 - 1900, 1902; Borradaile, piocladia, Caulerpa and Chondrococcus constitute more 1903; Betts, 1930 and Burton, 1940). Obser than 75% and are potentially useful sea weeds. vations of a general nature on fish and fisheiies of the Altogether 82 species of sea weeds were collected during islands were subsequently made by Ayyangar (1922), the survey, of which only 60 species are found in esti Mathew and Ramachandran (1956) and Balan (1958). mable quantities. However, published information on the marine living resources of the Lakshadweep, other than those of tunas and corals are rather scattered and scanty. While the major commercially important resources like the corals, tunas and other fish resources of the archipelago are dealt with elsewhere in this volume, here the potential ancillary resources are touched upon. -

B a S I C S T a T I S T I
ADMINISTRATION OF THE BASIC STATISTICS - 2014 UNION TERRITORY OF LAKSHADWEEP DIRECTORATE OF PLANNING & STATISTICS PRINTED AT LGP KAVARATTI UNION TERRITORY OF LAKSHADWEEP BASIC STATISTICS 2014 BY DIRECTORATE OF PLANNING & STATISTICS, SECRETARIAT, LAKSHADWEEP, KAVARATTI Phone No. 04896-262683, 263068 (Per & Fax), 263299 Email: [email protected] Kavaratti 11-04-2015 Kavaratti 11-04-2015 Kavaratti 16-04-2015 DISTANCE BETWEEN ISLANDS AND ISLANDS TO MAINLAND PORTS ( In Kilometres) (In nautical miles) Kochi Kochi Mang- Mang- alore 359 194 alore Calicut 219 330 178 118 Calicut And 228 257 293 158 139 123 And Amn 107 343 307 407 220 166 185 58 Amn Kdt 11 119 320 294 407 220 159 173 64 6 Kdt ix Btr 72 80 183 404 333 483 261 180 218 99 43 39 Btr Cht 56 54 65 141 348 269 432 233 145 188 76 35 29 30 Cht Klt 37 89 48 59 102 296 252 394 213 136 160 55 32 26 48 20 Klt Agt 111 104 80 72 61 157 383 363 459 248 196 207 85 33 39 43 56 60 Agt Kvt 57 115 126 124 76 65 119 346 352 404 218 190 187 64 35 41 67 68 62 31 Kvt Suh 54 81 169 176 167 126 135 165 407 419 446 241 226 220 89 62 73 90 96 91 44 29 Suh Klp 141 124 178 176 206 228 159 150 87 263 343 287 155 185 142 47 81 85 123 111 95 96 67 76 Klp Mcy 211 219 252 296 346 378 376 330 311 293 444 537 398 215 290 240 158 168 178 203 204 187 160 136 118 114 Mcy TECHNICAL GUIDANCE Director (Planning & Statistics) Shri. -

6-Gny-Nov-Dec-2012.Pdf
60 ` A Development and Environment Magazine Vol. 12, IssueVol. 12, 75, November-December 2012 www.geographyandyou.com Livelihood and Changing Social Values Coral Bleaching Seagrass Meadows of Lakshadweep Ocean Technology for Islands Tuna Fishing THE WHITE SANDS OF India The Lakshadweep Islands 120- 200 nautical miles off the south west coast of India, comprises of 11 inhabited islands (0.58 - 4.8 sq km in size ) and 16 uninhabited islands/islets; 6 newly formed/submerged banks and 3 coral reefs. The largest island is Minicoy with a length of about 9 km and area of 4.37 sq km. The total land availability including the inhabited and the uninhabited islands together is 32 sq km. Except Andrott, all other islands are protected by a nearly crescent shaped ‘reef wall’ with more than two boat channels. Known as the Laccadive, Aminidivi and Minicoy group of islands when it was formed into a Union Territory (UT) in 1956, it was renamed as Lakshadweep in 1973. These Islands are considered the smallest UT of India - however bestowed with 20,000 sq km of territorial waters and 40,000 sq km of exclusive economic zone - Lakshadweep is anything but small. Livelihood opportunities are very limited in the Lakshadweep Islands with Bitra Chetlat Kiltan Kadmat Bangaram Amini Agatti Andrott Kavaratti Kalpeni The inhabited islands of Minicoy Lakshadweep Map not to scale ISLANDSTHE LAKSHADWEEP deep sea and reef fishing as the main occupation followed by coconut cultivation. GEOGRAPHY AND YOU contents VOL. 12 ISSUE 75 NOVEMBER-DECEMBER 2012 KAVARATTI, LAKSHADWEEP According to provisional figures of 2011 Census, Lakshadweep stands in second place, ranking just below Kerala (93.91 per cent ) with an overall literacy rate of 92.28 per cent which is 5.58 per cent higher than that recorded during 2001. -

Coral Atolls of Lakshadweep, Arabian Sea, Indian Ocean
MOJ Ecology & Environmental Science Research Article Open Access Coral atolls of Lakshadweep, Arabian Sea, Indian Ocean Abstract Volume 2 Issue 2 - 2017 The Lakshadweep are least studied coral atoll group situated in the northern part of the Laccadive-Chagos ridge at a distance of 200-300km from the West Coast of India... Tapas Kumar Mallik The islands in the eastern side have lagoons which vary in size and are the habitat for Former Director, G.S.I, Marine Wing, India a variety of plants and aquatic animals. They are also sites for harbor, aquaculture, Tapas Kumar Mallik, Former Director, G.S.I, recreation and tourism. The smaller lagoons are virtually filled with sediments The Correspondence: Marine Wing, FD- 317, Sector-Iii, Salt Lake, Kolkata- 700106, larger lagoons are comparatively deep with depth of about 10 to 16m. At low tide India, Email [email protected] the reef is exposed and during high tide it is submerged. Eastern seaside part of the island has steep storm beach with deposits of shingles and boulders .and the lagoon Received: September 27, 2016 | Published: April 20, 2017 side beach is sandy. The source of the sediments on the atolls is the coral reefs and the other biological communities. A substantial amount of the sediments in the reef front is lost in the deep sea because of morphology, waves and currents. The inner reef flat has thicker sediment cover, parts of which are transported to the lagoon. The reef areas are the living places of various communities of corals belonging to different families of Acroporidae, Poritidae, Pocilloporidae, Favidae, Fungiidae, Mussidae etc. -

Reserve Bank of India Act, 1934
RESERVE BANK OF INDIA ACT, 1934 (As modified up to February 28, 2009) Disclaimer: Though the Reserve Bank of India has taken efforts to ensure the accuracy of the text as well as the footnotes of the Act, it will not be responsible in any way if any inadvertent mistake has crept in into the document. Any person relying on this document would be doing so at his own risk and the Reserve Bank would not be responsible for any consequences that may ensue such reliance. If any doubt occurs or discrepancy found, the reader is advised to refer to the Gazette copy of the relevant text. Any suggestion for correction or modification is however definitely welcome. Table of Contents CHAPTER I - PRELIMINARY 1. Short title, extent and commencement. 2. Definitions. CHAPTER II - INCORPORATION, CAPITAL, MANAGEMENT AND BUSINESS 3. Establishment and incorporation of Reserve Bank. 4. Capital of the Bank. 5. Increase and reduction of share capital. 6. Offices, branches and agencies. 7. Management. 8. Composition of the Central Board, and term of office of Directors. 9. Local Boards, their constitution and functions. 10. Disqualifications of Directors and members of Local Boards. 11. Removal from and vacation of office. 12. Casual Vacancies and absences 13. Meetings of the Central Board. 14 to 16. [General meetings. First constitution of the Central Board. First constitution of Local Board.] 17. Business which the Bank may transact. 18. Power of direct discount. 18A. Validity of loan or advance not to be questioned. 19. Business which the Bank may not transact. CHAPTER III - CENTRAL BANKING FUNCTIONS 20. -

Government of India India Meteorological Department Regional Meteorological Centre Mumbai – 400005 Press Release Dated: 31/10/2019 Time of Issue: 1800 Hrs
Government of India India Meteorological Department Regional Meteorological Centre Mumbai – 400005 Press Release Dated: 31/10/2019 Time of Issue: 1800 hrs. IST Formation of Severe Cyclonic Storm MAHA (Pronounced as M’maha) over eastcentral Arabian Sea and adjoining Lakshadweep area & weakening of Cyclonic Storm ‘Kyarr’ over westcentral Arabian Sea. 1. Severe Cyclonic Storm MAHA (Pronounced as M’maha) over eastcentral Arabian Sea and adjoining Lakshadweep area The Cyclonic storm MAHA (Pronounced as M’maha ) over Lakshadweep and adjoining southeast Arabian Sea moved further north-northwestwards with a speed of 17 kmph during past 06 hours, intensified into a Severe Cyclonic Storm and lay centered at 1130 hrs IST of 31st October, 2019 near latitude 11.9°N and longitude 72.8°E over eastcentral Arabian Sea and adjoining Lakshadweep area about 90 km north-northeast of Aminidivi (Lakshadweep), 150 km north-northeast of Kavaratti (Lakshadweep), 25 km east-northeast of Chetlat (Lakshadweep) and 330 km west-northwest of Kozhikode (Kerala). It is very likely to continue to move north- northwestwards across north Lakshadweep Islands during next 06 hours. Then, it is very likely to move northwestwards during subsequent 12 hours and thereafter move west- orthwestwards. It is very likely to intensify into a very severe cyclonic storm over eastcentral Arabian Sea during 24 hours. 2. Weakening Cyclonic Storm ‘Kyarr’ over westcentral Arabian Sea The Cyclonic Storm ‘Kyarr’ over westcentral Arabian Sea moved south-southwestwards with a speed of 13 Kmph during past 06 hrs and lay centred at 1130 hrs IST of 31st October, 2019 over westcentral Arabian Sea near latitude 17.7°N and longitude 60.1°E, about 1350 km west- southwest of Mumbai (Maharashtra), 640 km east-northeast of Salalah (Oman) and 350 km south-southeast of Masirah (Oman).