What's Going on with These Lithium Reagents?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Direct Preparation of Some Organolithium Compounds from Lithium and RX Compounds Katashi Oita Iowa State College
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1955 Direct preparation of some organolithium compounds from lithium and RX compounds Katashi Oita Iowa State College Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Organic Chemistry Commons Recommended Citation Oita, Katashi, "Direct preparation of some organolithium compounds from lithium and RX compounds " (1955). Retrospective Theses and Dissertations. 14262. https://lib.dr.iastate.edu/rtd/14262 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand comer and continuing from left to right in equal sections with small overiaps. -

And C,N-Chelated Organocopper Compounds†
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 2 September 2021 Review C,C- and C,N-Chelated Organocopper Compounds† Liang Liu1, Hui Chen2, Zhenqiang Yang2, Junnian Wei1* and Zhenfeng Xi1,3* 1 Beijing National Laboratory for Molecular Sciences (BNLMS), Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry, Peking University, Beijing 100871, China; [email protected] (L.L.), [email protected] (J.W.), [email protected] (Z.X.) 2 Henan Institute of Chemistry Co. Ltd., Henan Academy of Sciences, Zhengzhou 450002, China; [email protected] (H.C.), [email protected] (Z.Y.) 3 State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry (SIOC), Shanghai 200032, China; [email protected] (Z.X.) * Correspondence: [email protected] (J.W.), [email protected] (Z.X.) † Dedicated to Professor Gerard van Koten on the occasion of his 80th birthday Abstract: Copper-catalyzed and organocopper-involved reactions are of great significance in organic synthesis. To have a deep understanding of the reaction mechanisms, the structural characterizations of organocopper intermediates become indispensable. Meanwhile, the structure- function relationship of organocopper compounds would advance rational design and development of new Cu-based reactions and organocopper reagents. Compared to the mono- carbonic ligand, the C,N- and C,C-bidentate ligands better stabilize the unstable organocopper compounds. The bidentate ligands can chelate to the same copper atom via 휂2-mode, forming a mono-cupra-cyclic compounds with at least one acute C-Cu-C angle. When the bidentate ligands bind to two copper atoms via 휂1-mode at each coordinating site, the bimetallic macrocyclic compounds will form nearly linear C-Cu-C angles. -

United States Patent (19) 11 Patent Number: 5,945,382 Cantegrill Et Al
US005.945382A United States Patent (19) 11 Patent Number: 5,945,382 Cantegrill et al. (45) Date of Patent: *Aug. 31, 1999 54 FUNGICIDAL ARYLPYRAZOLES 2300173 12/1990 Japan. 2224208 5/1990 United Kingdom. 75 Inventors: Richard Cantegril, Lyons; Denis Croisat, Paris; Philippe Desbordes, OTHER PUBLICATIONS Lyons, Francois Guigues, English translation of JP 2-300173, 1990. Rillieux-la-Pape; Jacques Mortier, La English translation of JP 59–53468, 1984. Bouéxier; Raymond Peignier, Caluire; English translation of JP 3-93774, 1991. Jean Pierre Vors, Lyons, all of France Miura et al., (CA 1.14:164226), 1991. Miura et al., (CA 115:92260), 1991. 73 Assignee: Rhone-Poulenc Agrochimie, Lyons, Chemical Abstracts, vol. 108, No. 23, 1986, abstract No. France 204577b. CAS Registry Handbook, No. section, RN=114913-44-9, * Notice: This patent is subject to a terminal dis 114486-01-0, 99067-15-9, 113140-19-5, 73227-97-1, claimer. 27069-17-6, 18099-21–3, 17978-27-7, 1988. 21 Appl. No.: 08/325,283 Hattori et al., CA 68:68981 (1968), Registry No. 17978–25–5, 17978-26-6, 17978-27-7 and 18099–21-3. 22 PCT Filed: Apr. 26, 1993 Hattori et al., CA 68:68982 (1968), Registry No. 17978-28-8. 86 PCT No.: PCT/FR93/00403 Janssen et al., CA 78: 159514 (1973), Registry No. S371 Date: Dec. 22, 1994 38858-97-8 and 38859-02-8. Chang et al., CA 92:146667 (1980), Registry No. S 102(e) Date: Dec. 22, 1994 73227 91-1. Berenyi et al., CA 94:156963 (1981), Registry No. -

Conversion of Carbon Dioxide to Acetylene on a Micro Scale
810 NATURE June 14, 1947 Vol. 159 orbitale of the ethmoid is reduced". In the orang Stainless steel was found to be the most satis and the gibbon a large planum orbitale articulates factory furnace material tried. From mild steel in front with the lacrimal, as in man. The figure relatively large amounts of acetylene were produced we give of the orbital wall in Pleaianthropus shows in blank experiments, and a fused silica envelope a condition almost exactly as in man. fitted with a nickel thimble was found, after it had We are here not at present concerned with the been used with calcium and barium metals, to absorb question of whether man and the Australopithecinre carbon dioxide when hot even when no calcium or have arisen from an early anthropoid, or a pre barium was present. In carrying out the absorption anthropoid, or an Old World monkey or a tarsioid ; of carbon dioxide by barium metal in the stainless but we think the evidence afforded by this new skull steel furnace it was found that when the pressure of Plesianthropus shows that the Australopithecinre at which the gas was admitted was less than about and man are very closely allied, and that these small 10·1 mm. of mercury, the yield of acetylene was brained man-like beings were very nearly human. variable and only about 45 per cent. Good yields R. BROOM were obtained when the carbon dioxide at its full J. T. RoBINSON pressure was admitted to the furnace before raising Transvaal Museum, Pretoria. the temperature above 400° C. -
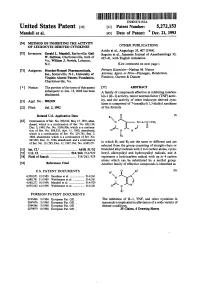
ESSEE in Which R1 and R2 Are the Same Or Different And
USOO5272153A United States Patent (19) 11 Patent Number: 5,272,153 Mandell et al. 45 Date of Patent: Dec. 21, 1993 (54)54 OFMETH E. NHIBITINSi6. CTVTY OTHER PUBLICATIONS Avido et al., Angiology 35, 407 (1984). 75 Inventors: St. Mel ES Suguira et al., Japanese Journal of Anesthesiology 32, va.8 William,Wan, CharlotteSVlie, Novici, Lebanon, O 435-41,35-41, with English translationtranslation. N.J. (List continued on next page.) 73) Assignees: Hoechst-Roussel Pharmaceuticals, Primary Examiner-Nathan M. Nutter Inc., Somerville, N.J.; University of Attorney, Agent, or Firm-Finnegan, Henderson, Virginia Alumni Patents Foundation, Farabow, Garrett & Dunner Charlottesville, Va. (*) Notice: The portion of the term of this patent 57) ABSTRACT E. to Jun 15, 2008 has been A family of compounds effective in inhibiting interleu SC. kin-1 (IL-1) activity, tumor necrosis factor (TNF) activ (21) Appl. No.: 908,929 ity, and the activity of other leukocyte derived cyto s kines is comprised of 7-(oxoalkyl) 1,3-dialkyl xanthines (22 Filed: Jul. 2, 1992 of the formula Related U.S. Application Data R (I) 63 Continuation of Ser. No. 700,522, May 15, 1991, aban- Y N-A-C-CH3 doned, which is a continuation of Ser. No. 622,138, Dec. 5, 1990, Pat. No. 5,096,906, which is a continua- al- 6 tion of Ser. No. 508,535, Apr. 11, 1990, abandoned, O N N which is a continuation of Ser. No. 239,761, Sep. 2, 1988, abandoned, which is a continuation of ser. No. R2 ESSEE selectedin which from R1 and the R2group are consistingthe same orof different straight-chain and are or 51) int. -

OMLI041 GHS US English US
OMLI041 - METHYLLITHIUM, 3M in diethoxymethane METHYLLITHIUM, 3M in diethoxymethane Safety Data Sheet OMLI041 Date of issue: 10/04/2017 Revision date: 05/24/2018 Version: 1.1 SECTION 1: Identification 1.1. Identification Product name : METHYLLITHIUM, 3M in diethoxymethane Product code : OMLI041 Product form : Mixture Physical state : Liquid Formula : CH3Li Synonyms : LITHIUM METHIDE Chemical family : METAL ALKYL 1.2. Recommended use and restrictions on use Recommended use : Chemical intermediate 1.3. Supplier GELEST, INC. 11 East Steel Road Morrisville, PA 19067 USA T 215-547-1015 - F 215-547-2484 - (M-F): 8:00 AM - 5:30 PM EST [email protected] - www.gelest.com 1.4. Emergency telephone number Emergency number : CHEMTREC: 1-800-424-9300 (USA); +1 703-527-3887 (International) SECTION 2: Hazard(s) identification 2.1. Classification of the substance or mixture GHS-US classification Flammable liquids Category 2 H225 Highly flammable liquid and vapor Pyrophoric liquids Category 1 H250 Catches fire spontaneously if exposed to air Substances and mixtures which in contact with water emit flammable H260 In contact with water releases flammable gases which may ignite gases Category 1 spontaneously Skin corrosion/irritation Category 1B H314 Causes severe skin burns and eye damage Serious eye damage/eye irritation Category 1 H318 Causes serious eye damage Specific target organ toxicity (single exposure) Category 3 H335 May cause respiratory irritation Full text of H statements : see section 16 2.2. GHS Label elements, including precautionary statements GHS US labeling Hazard pictograms (GHS US) : Signal word (GHS US) : Danger Hazard statements (GHS US) : H225 - Highly flammable liquid and vapor H250 - Catches fire spontaneously if exposed to air H260 - In contact with water releases flammable gases which may ignite spontaneously H314 - Causes severe skin burns and eye damage H318 - Causes serious eye damage H335 - May cause respiratory irritation Precautionary statements (GHS US) : P280 - Wear protective gloves/protective clothing/eye protection/face protection. -

United States Patent (19) 11 Patent Number: 6,057,352 Brown Et Al
US006057352A United States Patent (19) 11 Patent Number: 6,057,352 Brown et al. (45) Date of Patent: May 2, 2000 54 FUNGICIDAL CYCLIC AMIDES WO 96/17851 6/1996 WIPO ............................... CO7F 7/08 WO 96/26.191 8/1996 WIPO ... ... CO7D 249/12 75 Inventors: Richard James Brown, Newark, Del.: WO 96/36633 11/1996 WIPO ... ... CO7D 405/04 Deborah Ann Frasier, Martinez, Calif.; WO96/36229 11/1996 WIPO ... ... A01N 43/653 Michael Henry Howard, Jr., WO 97/02255 1/1997 WIPO m CO7D 261/12 Rockland; Gerard Michael Koether, OTHER PUBLICATIONS Bear, both of Del. Zvilichovsky, G., J. Heterocyclic Chem., 24,465–470, 1987. 73 Assignee: E. I. du Pont de Nemours and Zvilichovsky, G. et al., J. Heterocyclic Chem., 25, Company, Wilmington, Del. 1307-1310, 1988. Davis, M. et al., Australian J. Chem., 30(8), 1815-1818, 21 Appl. No.: 08/952,380 1977. 22 PCT Filed: May 8, 1996 Primary Examiner Mukund J. Shah 86 PCT No.: PCT/US96/06534 Assistant Examiner Deepak R. Rao S371 Date: Nov. 13, 1997 57 ABSTRACT S 102(e) Date: Nov. 13, 1997 Compounds of Formula (I), 87 PCT Pub. No.: WO96/36616 (I) PCT Pub. Date: Nov. 21, 1996 1.Y. Nz Related U.S. Application Data x - W 63 Continuation-in-part of application No. 08/442,433, May NY 17, 1995, abandoned. A-N 60 Provisional application No. 60/004,183, Sep. 22, 1995. Ye 51) Int. Cl." ...................... C07D 249/12; CO7D 233/30; AO1N 43/74; AO1N 43/56 and their N-oxides and agriculturally Suitable Salts are 52 U.S. -

Download Download
— Studies on Lithium Acetylide Kenneth N. Campbell and Barbara K. Campbell, The University of Notre Dame In contrast to the large amount of work done on the acetylene derivatives of sodium, potassium and calcium, little attention has been paid to the analogous compounds of lithium. In 1898 Moissani prepared lithium acetylide on a small scale, by the action of acetylene on a liquid ammonia solution of lithium. He reported that lithium acetylide was less soluble in liquid ammonia than sodium acetylide, and that when isolated from the solvent, it was less stable, undergoing decomposition with evolu- tion of acetylene. On the basis of the weight of lithium acetylide obtained from a given weight of lithium, and from the amount of acetylene liberated on hydrolysis, he assigned to lithium acetylide the formula C2Li2.C2H2.2NH3. Since that time no references to lithium acetylide or lithium alkylacetylides have appeared in the literature. It was the pur- pose of the present work, therefore, to prepare and analyze lithium acetylide and a lithium alkylacetylide, and to compare their reactions with those of the better known sodium derivatives. Experimental Procedure Preparation of Lithium and Sodium Acetylides.—Acetylene gas, washed by bubbling through concentrated sulfuric acid, was passed into two liters of liquid ammonia, while 7 g. (1 mole) of metallic lithium, cut in small pieces, was added gradually, with stirring, at a rate such that the solution did not develop a permanent deep blue color. When the solution became colorless after the addition of the last piece of lithium, the flow of acetylene was stopped. -

Nomination Background: Methyl Trans
' 7/?1 Methyl styryl ketone 122-57-6 NTP NOMINATION HISTORY AND REVIEW A. Nomination History 1. Nomination Source: NCI 2. Recommendations: -Comparative toxicity studies with methyl vinyl ketone -Metabolism -Carcinogenicity 3. Rationale/Remarks: -Potential for widespread human exposure -Nominated as a result of a class study of a,~-unsaturated ketones -Flavoring and fragrance additive -Natural product -Environmental pollutant -Mutagenic in Salmonella 4. Priority: -High for toxicity studies -Moderate for carcinogenicity s. Date of Nomination: July 1994 B. Interagency Committee for Chemical Evaluation and Coordination Review 1. Date of Review: 2. Recommendations: 3. Priority: 4. NTP Chemical Selection Principles: 5. Rationale/Remarks: 122-57-6 Methyl styryl ketone SUMMARY OF DATA FOR CHEMICAL SELECTION CHEMICAL IDENTIFICATION CAS Registry Number: 122-57-6 Chemical Abstracts Name: 3-Buten-2-one, 4-phenyl- (SCI, 9CI) Svnonvms and Trade Names: Acetocinnamone; benzalacetone; benzylideneacetone; methyl 2 phenylvinyl ketone; methyl styryl ketone; methyl ~-styryl ketone; 4 phenylbutenone; 4-phenyl-3-butene-2-one; 2-phenylvinyl methyl ketone; styryl methyl ketone; MSK FEMA Number: 2881 Related isomers CAS Registry Number: 937-53-1 Chemica] Abstracts Name: 3-Buten-2-one, 4-phenyl-, (Z)- (SCI, 9CI) Synonvms and Trade Names: cis -4-Phenyl-3-buten-2-one; cis -benzalacetone; cis benzylideneacetone; cis-methyl styryl ketone CAS Registry Number: 1896-62-4 Chemical Abstracts Name: 3-Buten-2-one, 4-phenyl-, (E)- (SCI, 9CI) Synonyms and Trade Names: trans-4-Phenyl-3-buten-2-one; trans-benzalacetone; trans benzylideneacetone; trans-methyl styryl ketone; TPBO Structure. Molecular Formula. and Molecular WeiKbt 0 II H•CHCCH3 Mol. wt.: 146.19 Chemical and Phvsical Properties (from Aldrich Chemical Co., 1993, 1994; Budavari, 1989) Description: White to yellow solid with a sweet, pungent, creamy, floral odor Prepared for NCI by Technical Resources, Inc. -

Stable Lithium Diisopropylamide and Method of Preparation
Europaisches Patentamt J European Patent Office Publication number: 0 205 583 Office europeen des brevets B1 EUROPEAN PATENT SPECIFICATION (45) Date of publication of patent specification: 30.01.91 Intel.5: C 07 C 211/65 (3) Application number: 86900522.3 @ Date of filing: 17.12.85 (8) International application number: PCT/US85/02509 ® International publication number: WO 86/03744 03.07.86 Gazette 86/14 STABLE LITHIUM DIISOPROPYLAMIDE AND METHOD OF PREPARATION. (M) Priority: 24.12.84 US 685318 Proprietor: LITHIUM CORPORATION OF AMERICA, INC. Post Office Box 795 Date of publication of application: Bessemer City, NC 28016 (US) 30.12.86 Bulletin 86/52 Inventor: MORRISON, Robert, Charles Publication of the grant of the patent: 1946 Elmwood Drive 30.01.91 Bulletin 91/05 Gastonia, NC 28054 (US) Inventor: HALL, Randy, Winf red Route 4 Box 697 (M) Designated Contracting States: Kings Mountain, NC 28086 (US) AT BE CH DE FR GB IT LI LU NL SE Inventor: RATHMAN, Terry, Lee 3843 Gardner Park Drive Gastonia, NC 28054 (US) References cited: US-A-3197 516 US-A-3 694516 US-A-3388178 US-A-4 006187 Representative: Gore, Peter Manson et al US-A-3446 860 US-A-4399 078 W.P. THOMPSON & CO. Coopers Building Church Street JOURNAL OF ORGANOMETALLIC CHEMISTRY, Liverpool L1 3AB (GB) vol. 4, 1965; GILMAN et al.: "Stabilities of some n-alkyllithium compounds in mixed solvent CO I References cited: 00 systems", pp. 483-487 JOURNAL OF ORGANOMETTALIC CHEMISTRY, m JOURNAL OF AMERICAN CHEMICAL SOCIETY, vol. 29, 1971; HONEYCUTT: "Kinetics of the in vol. -

Safe Handling of Organolithium Compounds in the Laboratory
FEATURE Safe handling of organolithium compounds in the laboratory Organolithium compounds are extremely useful reagents in organic synthesis and as initiators in anionic polymerizations. These reagents are corrosive, flammable, and in certain cases, pyrophoric. Careful planning prior to execution of the experiment will minimize hazards to personnel and the physical plant. The proper personal protective equipment (PPE) for handling organolithium compounds will be identified. Procedures to minimize contact with air and moisture will be presented. Solutions of organolithium compounds can be safely transferred from the storage bottles to the reaction flask with either a syringe or a cannula. With the utilization of these basic techniques, organolithium compounds can be safely handled in the laboratory. By James A. Schwindeman, oxides (typi®ed by lithium t-butoxide). various classes of organolithium com- Chris J. Woltermann, and These organolithium compounds have pounds, with pKa from 15.2 (lithium Robert J. Letchford found wide utility as reagents for methoxide) to 53 (t-butyllithium).5 organic synthesis in a variety of appli- Fourth, organolithium reagents demon- cations. For example, they can be strate enhanced nucleophilicity com- INTRODUCTION employed as strong bases (alkyl- pared to the corresponding organo- When properly handled, organo- lithiums, aryllithiums, lithium amides magnesium compound. Finally, they lithiums provide unique properties and lithium alkoxides), nucleophiles are convenient, as a variety of organo- that allow for -

Lithium Diisopropylamide: Nonequilibrium Kinetics and Lessons Learned About Rate Limitation Russell F
Perspective pubs.acs.org/joc Lithium Diisopropylamide: Nonequilibrium Kinetics and Lessons Learned about Rate Limitation Russell F. Algera, Lekha Gupta, Alexander C. Hoepker, Jun Liang, Yun Ma, Kanwal J. Singh, and David B. Collum* Department of Chemistry and Chemical Biology Baker Laboratory, Cornell University, Ithaca, New York 14853-1301, United States *S Supporting Information ABSTRACT: The kinetics of lithium diisopropylamide (LDA) in tetrahydrofuran under nonequilibrium conditions are reviewed. These conditions correspond to a class of substrates in which the rates of LDA aggregation and solvation events are comparable to the rates at which various fleeting intermediates react with substrate. Substrates displaying these reactivities, by coincidence, happen to be those that react at tractable rates on laboratory time scales at −78 °C. In this strange region of nonlimiting behavior, rate-limiting steps are often poorly defined, sometimes involve deaggregation, and at other times include reaction with substrate. Changes in conditions routinely cause shifts in the rate-limiting steps, and autocatalysis is prevalent and can be acute. The studies are described in three distinct portions: (1) methods and strategies used to deconvolute complex reaction pathways, (2) the resulting conclusions about organolithium reaction mechanisms, and (3) perspectives on the concept of rate limitation reinforced by studies of LDA in tetrahydrofuran at −78 °C under nonequilibrium conditions. ithium diisopropylamide (LDA), a highly reactive and at −78 °Cconditions that are of singular importance in L selective Brønsted base, stands among the most prominent organic synthesisoccur at nearly the rates at which the reagents in organic synthesis.1 A survey of 500 total syntheses aggregates in Scheme 1 exchange.