Lake-Sediment Record of PAH, Mercury, and Fly-Ash Particle Deposition Near Coal-Fired Power Plants in Central Alberta, Canada
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Jackfish Lake State of the Watershed Report
Jackfish Lake State of the Watershed Report April 2016 North Saskatchewan Watershed Alliance 202 - 9440 49th St NW Edmonton, AB T6B 2M9 Tel: (587) 525-6820 Email: [email protected] http://www.nswa.ab.ca The North Saskatchewan Watershed Alliance (NSWA) is a non-profit society whose purpose is to protect and improve water quality and ecosystem functioning in the North Saskatchewan River watershed in Alberta. The organization is guided by a Board of Directors composed of member organizations from within the watershed. It is the designated Watershed Planning and Advisory Council (WPAC) for the North Saskatchewan River under the Government of Alberta’s Water for Life Strategy.. This report was prepared by Jennifer Regier, B.Sc. and David Trew, P.Biol of the North Saskatchewan Watershed Alliance. Cover photo credit: Dara Choy, Stony Plain AB Suggested Citation: North Saskatchewan Watershed Alliance (NSWA). 2016. Jackfish Lake State of the Watershed Report. Prepared by the NSWA, Edmonton, AB. for the Jackfish Lake Management Association, Carvel, AB. Available on the internet at http://www.nswa.ab.ca/resources/ nswa_publications Jackfish Lake State of the Watershed Report Executive Summary The purpose of this report is to consolidate environmental information on Jackfish Lake and its watershed in an effort to support future planning and management discussions. The report provides perspective on current environmental conditions at the lake relative to regional and historic trends. The report is provided as advice to the Jackfish Lake Management Association (JLMA), Alberta Environment and Parks, and Parkland County. The technical information contained in this document is detailed and addresses many lake and watershed features. -

Wabamun Lake Water Quality 1982 to 2001
WABAMUN LAKE WATER QUALITY 1982 TO 2001 WABAMUN LAKE WATER QUALITY 1982 TO 2001 Prepared by: Richard Casey, M.Sc. Limnologist Science and Standards Alberta Environment September 2003 W0309 Pub. No: T/695 ISBN: 0-7785-2503-1 (Printed Edition) ISBN: 0-7785-2504-X (On-Line Edition) Web Site: http://www3.gov.ab.ca/env/info/infocentre/publist.cfm Any comments, questions, or suggestions regarding the content of this document may be directed to: Environmental Monitoring and Evaluation Branch Alberta Environment 10th Floor, Oxbridge Place 9820 – 106th Street Edmonton, Alberta T5K 2J6 Phone: (780) 427-6278 Fax: (780) 422-6712 Additional copies of this document may be obtained by contacting: Information Centre Alberta Environment Main Floor, Great West Life Building 9920 – 108th Street Edmonton, Alberta T5K 2M4 Phone: (780) 944-0313 Fax: (780) 427-4407 Email: [email protected] SUMMARY Wabamun Lake, approximately 60 km west of Edmonton, is large, shallow, and generally well mixed. Sport fish in the lake include northern pike, yellow perch, and lake whitefish. There are a unique mix of land uses in the lake watershed, which include undisturbed bush and forest, agriculture, two coal mines with active and reclaimed areas, three coal-fired power plants, major transportation (road and rail) corridors, residences, and recreation. The mines supply fuel for the power plants, operated by the TransAlta Utilities Corporation (TAU). Industrial wastewaters, runoff and cooling water from the Whitewood mine and Wabamun power plant are discharged to the lake. Over time, TAU operations associated with the mines and power plants in the watershed have caused cumulative and ongoing impacts on the lake level. -

Ca 1978 ISSS Tours 8+16E Report.Pdf
11th CONGRESS I NT ERNA TI ONAL I OF SOIL SCIENCE EDMONTON, CANADA JUNE 1978 GUIDEBOOK FOR A SOILS LAND USE TOUR IN BANFF AND JASPER NATIONAL PARKS TOURS 8 AND 16 L.J. KNAPIK Soils Division, Al Research Council, Edmonton G.M. COEN Research Branch, culture Canada, Edmonton Alberta Research Council Contribution Series 809 ture Canada Soil Research Institute tribution 654 Guidebook itors D.F. Acton and L.S. Crosson Saskatchewan Institute of Pedology Saskatoon, Saskatchewan ~-"-J'~',r--- --\' "' ~\>(\ '<:-q, ,v ~ *'I> co'"' ~ (/) ~ AlBERTA \._____ ) / ~or th '(<.\ ~ e r ...... e1Bowden QJ' - Q"' Olds• Y.T. I N.W.T. _...,_.. ' h./? 1 ...._~ ~ll"O"W I ,-,- B.C. / U.S.A. ' '-----"'/' FIG. 1 GENERAL ROUTE MAP i; i TABLE OF CONTENTS Page ACKNOWLEDGEMENTS ...............•..................................... vi INTRODUCTION ........................................................ 1 GENERAL ITINERARY ................................................... 2 REGIONAL OVERVIEW ..•................................................. 6 The Alberta Plain .................................................. 6 15 The Rocky Mountain Foothills ........................................ The Rocky Mountains ................................................ 17 DAY 1: EDMONTON TO BANFF . • . 27 Road Log No. 1: Edmonton to Calgary.......................... 27 The Lacombe Research Station................................. 32 Road Log No. 2: Calgary to Banff............................ 38 Kananaskis Site: Orthic Eutric Brunisol.... .. ...... ... ....... 41 DAY 2: BANFF AND -

North Saskatchewan Regional Plan: Lake Paleolimnology Survey Final Report
Contract Name: NSRP Lake Paleolimnology Survey Consultant Name: Hutchinson Environmental Sciences Ltd. This report was commissioned by Alberta Environment and Sustainable Resource Development (now Alberta Environment and Parks) to support both the implementation of the Land-Use Framework and the Cumulative Effects Management System. Recreational lakes within the North Saskatchewan Regional plan boundaries are of high ecological and recreational value. To better understand how to effectively manage cumulative impacts on the lakes, it is necessary to understand conditions in the lakes prior to development. Knowing these pre-development conditions will assist in setting reasonable and achievable goals for lake management. The paleolimnology study was undertaken by Hutchinson Environmental Sciences Ltd. The objective of the study was to provide a paleolimnological reconstruction of water quality conditions at Pigeon and Wabamun lakes. Sediment cores were collected, sectioned, and analyzed to assess temporal variability in paleolimnological indicators. Based on these results, an overview of anthropogenic impacts on lake water quality was to be provided. Both Wabamun and Pigeon lakes are productive, alkaline, and polymictic, and are situated within a carbonaceous geological setting. These characteristics are known to influence the interpretation of results and were not sufficiently considered in the report. Future work may be required to further describe the limitations and evaluate the data presented in report. This report has been completed in accordance with the contract issued by Alberta Environment and Parks (AEP). AEP has closed this project and considers this report final. AEP does not necessarily endorse all of the contents of this report, nor does the report necessarily represent the views or opinions of AEP or stakeholders. -

Mayatan Lake State of the Watershed Report
Mayatan Lake State of the Watershed Report August 2012 Mayatan Lake State of the Watershed Report North Saskatchewan Watershed Alliance 9504‐49 Street Edmonton, AB T6B 2M9 Tel: (780) 442‐6363 Fax: (780) 495‐0610 Email: [email protected] http://www.nswa.ab.ca The North Saskatchewan Watershed Alliance (NSWA) is a non‐profit society whose purpose is to protect and improve water quality and ecosystem functioning in the North Saskatchewan River watershed in Alberta. The organization is guided by a Board of Directors composed of member organizations from within the watershed. It is the designated Watershed Planning and Advisory Council (WPAC) for the North Saskatchewan River under the Government of Alberta’s Water for Life Strategy. This report was prepared by Melissa Logan, P.Biol., Billie Milholland, B.A., and David Trew, P.Biol., of the North Saskatchewan Watershed Alliance. Suggested Citation: North Saskatchewan Watershed Alliance (NSWA). 2012. Mayatan Lake State of the Watershed Report. Prepared by the NSWA, Edmonton, AB., for the Mayatan Lake Management Association, Carvel, AB. Available on the internet at http://www.nswa.ab.ca/resources/nswa_publications 1 Mayatan Lake State of the Watershed Report Acknowledgements The NSWA gratefully acknowledges the contribution of the following persons towards the completion of this report: Yaw Okyere and Janet Yan of Alberta Environment for conducting a search of water allocations within the basin; Terry Chamuluk of Alberta Environment for providing Morton monthly evaporation estimates for Alberta; Ron Woodvine of AAFC for providing precipitation and evaporation tables and maps for the Canadian Prairies; Rick Rickwood and Candace Vanin of AAFC for providing maps and delineating the gross and effective areas of Mayatan Lake; Graham Watt for preparing maps; and Sal Figliuzzi for completion of the water balance for the lake. -

Prepared For
Volume 5D, ESA – Trans Mountain Pipeline ULC Socio-Economic Technical Reports Trans Mountain Expansion Project Traditional Land and Resource Use Technical Report An Elder reported that he and fellow Ermineskin Cree Nation community members once fished for whitefish, pickerel, perch, rainbow trout, pike and bull trout in Wabamun Lake. However, due to an oil spill in 2005, the water quality is now poor and fishing is not ideal. The fish are small and are believed to be unhealthy due to pollution. Although Ermineskin Cree Nation community members do not travel to the lake to fish, community members from nearby bands still report it to be an important fishing site. Community members report that some of their past fishing sites are no longer used. An Elder identified Pigeon Lake as a fishing site (Plate 5.1.7-1). Most fishing takes place at the south end of the lake. Historically, net fishing has been conducted. Community members reported that Buck Lake was the best spot to catch whitefish in the past. Chimney Creek, near Kootenay Plains, was also a known fishing site, now used for grazing livestock and not often used by Ermineskin Cree Nation members. A cabin was once situated there. Plate 5.1.7-1 Pigeon Lake from helicopter overflight. TABLE 5.1.7-5 FISHING SITES IDENTIFIED BY ERMINESKIN CREE NATION Approximate Distance and Current/Past Requested Direction from Project Site Description Use Mitigation 31 km south of RK 15.4 Coal Lake Current None 51.8 km southwest of RK 29.9 Pigeon Lake Current None 24.6 km south of RK 61.4 Along North Saskatchewan River for Current None trout, sturgeon, rainbow trout, catfish, suckers and walleye. -

Lake Stewardship Reference Guide
Association of Summer Villages of Alberta Association of Summer Villages of Alberta Lake Stewardship ReferenceReference GuideGuide Association of Summer Villages of Alberta 2006 Edition Acknowledgements The Association of Summer Villages of Alberta (ASVA), representing Alberta’s Summer Village municipalities, was formed in 1977. The ASVA advocates on common Summer Village issues, proposes solutions, and provides education for Summer Village Councils. The ASVA has long maintained that every Summer Village is dealing with lake stewardship issues today, and will continue to deal with them in the future. Through this Lake Stewardship Reference Guide, the ASVA has taken a leadership role in the stewardship of our lakes in partnership with other provincial government departments, organizations, and agencies. The Lake Stewardship Reference Guide was prepared through the ASVA Lake Stewardship Steering Committee. Members of this Lake Stewardship Steering Committee include: Bruce McIntosh, Chair, Summer Village of Island Lake Susan Evans-Dzus, Summer Villages of Seba Beach and Lakeview Art Lamoureux, Summer Village of White Sands Bob Lindsay, Summer Village of Sunset Beach Lori Jeffery-Heaney, Summer Village of Val Quentin, Alberta Urban Municipalities Association Director of Summer Villages Jim Sandmaier, Summer Village of Island Lake South Peter Wright, Summer Village of Sunrise Beach Special thanks to the following individuals who participated in preparing this Reference Guide: Gerry Haekel, Alberta Sustainable Resource Development Shelleen Lakusta, Alberta Environment Christine Lazaruk, Alberta Urban Municipalities Association, Alberta Environment Terry Sly, Alberta Environment Bruce Thom, Alberta Urban Municipalities Association Island Lake (front cover) photo credit: Jim Sandmaier Contributing authors and editors: Pat Valastin and Lori Jeffery-Heaney Design and graphic layout services: Pat Valastin and Hound’s Tooth Communications Ltd. -
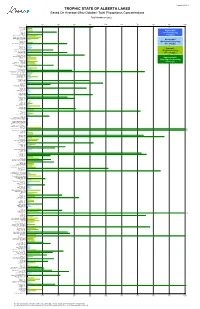
Trophic State of Alberta Lakes Based on Average Total Phosphorus
Created Feb 2013 TROPHIC STATE OF ALBERTA LAKES Based On Average (May-October) Total Phosphorus Concentrations Total Phosphorus (µg/L) 0 100 200 300 400 500 600 700 800 900 1000 * Adamson Lake Alix Lake * Amisk Lake * Angling Lake Oligotrophic * ‡ Antler Lake Arm Lake (Low Productivity) * Astotin Lake (<10 µg/L) * ‡ Athabasca (Lake) - Off Delta Baptiste Lake - North Basin Baptiste Lake - South Basin * ‡ Bare Creek Res. Mesotrophic * ‡ Barrier Lake ‡ Battle Lake (Moderate Productivity) * † Battle River Res. (Forestburg) (10 - 35 µg/L) Beartrap Lake Beauvais Lake Beaver Lake * Bellevue Lake Eutrophic * † Big Lake - East Basin * † Big Lake - West Basin (High Productivity) * Blackfalds Lake (35 - 100 µg/L) * † Blackmud Lake * ‡ Blood Indian Res. Bluet (South Garnier Lake) ‡ Bonnie Lake Hypereutrophic † Borden Lake * ‡ Bourque Lake (Very High Productivity) ‡ Buck Lake (>100 µg/L) Buffalo Lake - Main Basin Buffalo Lake - Secondary Bay * † Buffalo Lake (By Boyle) † Burntstick Lake Calling Lake * † Capt Eyre Lake † Cardinal Lake * ‡ Carolside Res. - Berry Creek Res. † Chain Lakes Res. - North Basin † Chain Lakes Res.- South Basin Chestermere Lake * † Chickakoo Lake * † Chickenhill Lake * Chin Coulee Res. * Clairmont Lake Clear (Barns) Lake Clear Lake ‡ Coal Lake * ‡ Cold Lake - English Bay ‡ Cold Lake - West Side ‡ Cooking Lake † Cow Lake * Crawling Valley Res. Crimson Lake Crowsnest Lake * † Cutbank Lake Dillberry Lake * Driedmeat Lake ‡ Eagle Lake ‡ Elbow Lake Elkwater Lake Ethel Lake * Fawcett Lake * † Fickle Lake * † Figure Eight Lake * Fishing Lake * Flyingshot Lake * Fork Lake * ‡ Fox Lake Res. Frog Lake † Garner Lake Garnier Lake (North) * George Lake * † Ghost Res. - Inside Bay * † Ghost Res. - Inside Breakwater ‡ Ghost Res. - Near Cochrane * Gleniffer Lake (Dickson Res.) * † Glenmore Res. -

Western Grebe Surveys in Alberta 2016
WESTERN GREBE SURVEYS IN ALBERTA 2016 The western grebe has been listed as a Threatened species in Alberta. A recent data compilation shows that there are approximately 250 lakes that have supported western grebes in Alberta. However, information for most lakes is poor and outdate d. Total counts on lakes are rare, breeding status is uncertain, and the location and extent of breeding habitat (emergent vegetation, usually bulrush) is usually unknown. We are seeking your help in gathering more information on western grebe populations in Alberta. If you visit any of the lakes listed below, or know anyone that does, we would appreciate as much detail as you can collect on the presence of western grebes and their habitat. Let us know in advance (if possible) if you are planning on going to any lakes, and when you do, e-mail details of your observations to [email protected]. SURVEY METHODS: Visit a lake between 1 May and 31 August with spotting scope or good binoculars. Surveys can be done from a boat, or vantage point(s) from shore. Report names of surveyors, dates, number of adults seen, and report on the approximate percentage of the lake area that this number represents. Record presence of young birds or nesting colonies, and provide any additional information on presence/location of likely breeding habitat, specific parts of the lake observed, observed threats to birds or habitat (boat traffic, shoreline clearing, pollution, etc.). Please report on findings even if no birds were seen. Lakes on the following page that are flagged with an asterisk (*) were not visited in 2015, and are priority for survey in 2016. -

Monitoring for Invasive Mussels in Alberta’S Irrigation Infrastructure: 2017 Report
Monitoring for Invasive Mussels in Alberta’s Irrigation Infrastructure: 2017 Report Alberta Agriculture and Forestry Water Quality Section Outlet of Sauder Reservoir January 2018 Introduction and Summary The Government of Alberta (GOA) is committed to protecting the province against aquatic invasive species (AIS), due to their negative ecological and economic effects. Invasive zebra mussels (Dreissena polymorpha) and quagga mussels (Dreissena bugensis) are of prominent concern, as these dreissenid mussels attach to any solid submerged surface and rapidly multiply due to their high reproductive rates. They are also very difficult to contain and eradicate once established. Additionally, they are spreading closer to Alberta’s borders. Alberta’s irrigation industry contributes $3.6 billion to the provincial gross domestic product (GDP). Specifically, it contributes about 20% of the provincial agri-food sector GDP on 4.7% of the province’s cultivated land base (Paterson Earth & Water Consulting 2015). Alberta’s irrigation industry includes thirteen irrigation districts that supply water to more than 570,000 ha of farmland through infrastructure valued at $3.6 billion. This infrastructure includes 57 irrigation reservoirs along with 3,491 km of canals and 4,102 km of pipelines (ARD 2014; AF 2017). The irrigation conveyance system provides water to irrigators, municipalities, industries, and wetlands, while the reservoirs support recreational activities such as boating and fishing and provide habitat to fish and waterfowl. Invasive mussels are a concern to the irrigation industry as infestations will have a significant negative effect on water infrastructure and conveyance works due to their ability to completely clog pipelines and damage raw-water treatment systems and intakes. -

Wildlife Management Unit 336 Aerial Ungulate Survey 2020
Aerial Wildlife Survey Report Wildlife Management Unit 336 Aerial Ungulate Survey (2020) Background Wildlife Management Unit (WMU) 336 (Wabamun) is located in west-central Alberta within the Red Deer-North Saskatchewan Region. The unit is located northeast of Drayton Valley and is bounded by Hwy 22 and the Pembina River to the west, and the North Saskatchewan River to the south. This 2616 km2 area has extensive open coal pit mines concentrated to the south of Wabamun Lake, but the majority of the WMU consists of mixed farming and rural residential lands. The most recent previous aerial ungulate survey conducted in this unit used the Gasaway survey method and was conducted in 2012. Results of previous surveys are summarized in Tables 1-3. The objective of the survey conducted in January, 2020 was to assess the status of the ungulate population in this WMU by determining estimates of population size, density and age-sex composition. We also recorded observations of additional species of interest, including carnivores, game birds, and sensitive or species at risk. Survey Method From January 16-20, 2020, a strip transect survey was conducted flying north-south lines at 1.6 km (1 mile) intervals resulting in 25% coverage (with 400 m wide survey strip) for a total linear survey effort of 1586 km (634 km2). Snow conditions were good and uniformly consistent throughout the unit. At each observation point, ungulates were counted by species, and, when possible, classified by sex and age. Sex and age classification was determined for moose by identification of vulva patch and size differential of cow/calves. -

Mercury in Fish 2009-2013
2016 Mercury in Fish In Alberta Water Bodies 2009–2013 For more information on Fish Consumption Advisories Contact: Health Protection Branch Alberta Health P.O. Box 1360, Station Main Edmonton, Alberta, T5J 1S6 Telephone: 1-780-427-1470 ISBN: 978-0-7785- 8283-0 (Report) ISBN: 978-0-7785- 8284-7 (PDF) 2016 Government of Alberta Alberta Health, Health Protection Branch Mercury in Fish in Alberta Water Bodies 2009 – 2013 February 2016 Executive Summary Mercury enters the environment through various natural processes and human activities. Methylmercury is transformed from inorganic forms of mercury via methylation by micro-organisms in natural waters, and can accumulate in some fish. Humans are exposed to very low levels of mercury directly from the air, water and food. Fish consumers may be exposed to relatively higher levels of methylmercury by eating mercury-containing fish from local rivers and lakes. Methylmercury can accumulate in the human body over time. Because methylmercury is a known neurotoxin, it is necessary to limit human exposure. From 2009 to 2013, the Departments of Environment and Parks (AEP) and Health (AH) initiated a survey of mercury levels in fish in selected water bodies in Alberta. These water bodies are extensively accessed by the public for recreational activities. This report deals with (1) concentrations of total mercury levels in various fish species collected from the water bodies in Alberta, (2) estimated exposures, (3) fish consumption limits, (4) fish consumption advisories, and (5) health benefits of fish consumption. The results indicate that: 1. Concentrations of total mercury in fish in the water bodies in Alberta were within the ranges for the same fish species from other water bodies elsewhere in Canada and the United States.