Analytical Method for 2,4-DP-P, 2,4-D, 2,4-DB, MCPA, MCPB, Mecoprop-P and Their 2- Ehs in Soil
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2,4-Dichlorophenoxyacetic Acid
2,4-Dichlorophenoxyacetic acid 2,4-Dichlorophenoxyacetic acid IUPAC (2,4-dichlorophenoxy)acetic acid name 2,4-D Other hedonal names trinoxol Identifiers CAS [94-75-7] number SMILES OC(COC1=CC=C(Cl)C=C1Cl)=O ChemSpider 1441 ID Properties Molecular C H Cl O formula 8 6 2 3 Molar mass 221.04 g mol−1 Appearance white to yellow powder Melting point 140.5 °C (413.5 K) Boiling 160 °C (0.4 mm Hg) point Solubility in 900 mg/L (25 °C) water Related compounds Related 2,4,5-T, Dichlorprop compounds Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) 2,4-Dichlorophenoxyacetic acid (2,4-D) is a common systemic herbicide used in the control of broadleaf weeds. It is the most widely used herbicide in the world, and the third most commonly used in North America.[1] 2,4-D is also an important synthetic auxin, often used in laboratories for plant research and as a supplement in plant cell culture media such as MS medium. History 2,4-D was developed during World War II by a British team at Rothamsted Experimental Station, under the leadership of Judah Hirsch Quastel, aiming to increase crop yields for a nation at war.[citation needed] When it was commercially released in 1946, it became the first successful selective herbicide and allowed for greatly enhanced weed control in wheat, maize (corn), rice, and similar cereal grass crop, because it only kills dicots, leaving behind monocots. Mechanism of herbicide action 2,4-D is a synthetic auxin, which is a class of plant growth regulators. -

Herbicide Mode of Action Table High Resistance Risk
Herbicide Mode of Action Table High resistance risk Chemical family Active constituent (first registered trade name) GROUP 1 Inhibition of acetyl co-enzyme A carboxylase (ACC’ase inhibitors) clodinafop (Topik®), cyhalofop (Agixa®*, Barnstorm®), diclofop (Cheetah® Gold* Decision®*, Hoegrass®), Aryloxyphenoxy- fenoxaprop (Cheetah®, Gold*, Wildcat®), fluazifop propionates (FOPs) (Fusilade®), haloxyfop (Verdict®), propaquizafop (Shogun®), quizalofop (Targa®) Cyclohexanediones (DIMs) butroxydim (Factor®*), clethodim (Select®), profoxydim (Aura®), sethoxydim (Cheetah® Gold*, Decision®*), tralkoxydim (Achieve®) Phenylpyrazoles (DENs) pinoxaden (Axial®) GROUP 2 Inhibition of acetolactate synthase (ALS inhibitors), acetohydroxyacid synthase (AHAS) Imidazolinones (IMIs) imazamox (Intervix®*, Raptor®), imazapic (Bobcat I-Maxx®*, Flame®, Midas®*, OnDuty®*), imazapyr (Arsenal Xpress®*, Intervix®*, Lightning®*, Midas®* OnDuty®*), imazethapyr (Lightning®*, Spinnaker®) Pyrimidinyl–thio- bispyribac (Nominee®), pyrithiobac (Staple®) benzoates Sulfonylureas (SUs) azimsulfuron (Gulliver®), bensulfuron (Londax®), chlorsulfuron (Glean®), ethoxysulfuron (Hero®), foramsulfuron (Tribute®), halosulfuron (Sempra®), iodosulfuron (Hussar®), mesosulfuron (Atlantis®), metsulfuron (Ally®, Harmony®* M, Stinger®*, Trounce®*, Ultimate Brushweed®* Herbicide), prosulfuron (Casper®*), rimsulfuron (Titus®), sulfometuron (Oust®, Eucmix Pre Plant®*, Trimac Plus®*), sulfosulfuron (Monza®), thifensulfuron (Harmony®* M), triasulfuron (Logran®, Logran® B-Power®*), tribenuron (Express®), -

INDEX to PESTICIDE TYPES and FAMILIES and PART 180 TOLERANCE INFORMATION of PESTICIDE CHEMICALS in FOOD and FEED COMMODITIES
US Environmental Protection Agency Office of Pesticide Programs INDEX to PESTICIDE TYPES and FAMILIES and PART 180 TOLERANCE INFORMATION of PESTICIDE CHEMICALS in FOOD and FEED COMMODITIES Note: Pesticide tolerance information is updated in the Code of Federal Regulations on a weekly basis. EPA plans to update these indexes biannually. These indexes are current as of the date indicated in the pdf file. For the latest information on pesticide tolerances, please check the electronic Code of Federal Regulations (eCFR) at http://www.access.gpo.gov/nara/cfr/waisidx_07/40cfrv23_07.html 1 40 CFR Type Family Common name CAS Number PC code 180.163 Acaricide bridged diphenyl Dicofol (1,1-Bis(chlorophenyl)-2,2,2-trichloroethanol) 115-32-2 10501 180.198 Acaricide phosphonate Trichlorfon 52-68-6 57901 180.259 Acaricide sulfite ester Propargite 2312-35-8 97601 180.446 Acaricide tetrazine Clofentezine 74115-24-5 125501 180.448 Acaricide thiazolidine Hexythiazox 78587-05-0 128849 180.517 Acaricide phenylpyrazole Fipronil 120068-37-3 129121 180.566 Acaricide pyrazole Fenpyroximate 134098-61-6 129131 180.572 Acaricide carbazate Bifenazate 149877-41-8 586 180.593 Acaricide unclassified Etoxazole 153233-91-1 107091 180.599 Acaricide unclassified Acequinocyl 57960-19-7 6329 180.341 Acaricide, fungicide dinitrophenol Dinocap (2, 4-Dinitro-6-octylphenyl crotonate and 2,6-dinitro-4- 39300-45-3 36001 octylphenyl crotonate} 180.111 Acaricide, insecticide organophosphorus Malathion 121-75-5 57701 180.182 Acaricide, insecticide cyclodiene Endosulfan 115-29-7 79401 -

Appendix a Analysis of Products with Two Or More Active Ingredients
APPENDIX A ANALYSIS OF PRODUCTS WITH TWO OR MORE ACTIVE INGREDIENTS The Agency does not routinely include, in its risk assessments, an evaluation of mixtures of active ingredients, either those mixtures of multiple active ingredients in product formulations or those in the applicator’s tank. In the case of the product formulations of active ingredients (that is, a registered product containing more than one active ingredient), each active ingredient is subject to an individual risk assessment for regulatory decision regarding the active ingredient on a particular use site. If effects data are available for a formulated product containing more than one active ingredient, they may be used qualitatively or quantitatively1 2. There are no product LD50 values, with associated 95% Confidence Intervals (CIs) available for glyphosate. As discussed in USEPA (2000) a quantitative component-based evaluation of mixture toxicity requires data of appropriate quality for each component of a mixture. In this mixture evaluation an LD50 with associated 95% CI is needed for the formulated product. The same quality of data is also required for each component of the mixture. Given that the formulated products for glyphosate do not have LD50 data available it is not possible to undertake a quantitative or qualitative analysis for potential interactive effects. However, because the active ingredients are not expected to have similar mechanisms of action, metabolites, or toxicokinetic behavior, it is reasonable to conclude that an assumption of dose-addition would be inappropriate. Consequently, an assessment based on the toxicity of glyphosate is the only reasonable approach that employs the available data to address the potential acute risks of the formulated products. -

List of Herbicide Groups
List of herbicides Group Scientific name Trade name clodinafop (Topik®), cyhalofop (Barnstorm®), diclofop (Cheetah® Gold*, Decision®*, Hoegrass®), fenoxaprop (Cheetah® Gold* , Wildcat®), A Aryloxyphenoxypropionates fluazifop (Fusilade®, Fusion®*), haloxyfop (Verdict®), propaquizafop (Shogun®), quizalofop (Targa®) butroxydim (Falcon®, Fusion®*), clethodim (Select®), profoxydim A Cyclohexanediones (Aura®), sethoxydim (Cheetah® Gold*, Decision®*), tralkoxydim (Achieve®) A Phenylpyrazoles pinoxaden (Axial®) azimsulfuron (Gulliver®), bensulfuron (Londax®), chlorsulfuron (Glean®), ethoxysulfuron (Hero®), foramsulfuron (Tribute®), halosulfuron (Sempra®), iodosulfuron (Hussar®), mesosulfuron (Atlantis®), metsulfuron (Ally®, Harmony®* M, Stinger®*, Trounce®*, B Sulfonylureas Ultimate Brushweed®* Herbicide), prosulfuron (Casper®*), rimsulfuron (Titus®), sulfometuron (Oust®, Eucmix Pre Plant®*), sulfosulfuron (Monza®), thifensulfuron (Harmony®* M), triasulfuron, (Logran®, Logran® B Power®*), tribenuron (Express®), trifloxysulfuron (Envoke®, Krismat®*) florasulam (Paradigm®*, Vortex®*, X-Pand®*), flumetsulam B Triazolopyrimidines (Broadstrike®), metosulam (Eclipse®), pyroxsulam (Crusader®Rexade®*) imazamox (Intervix®*, Raptor®,), imazapic (Bobcat I-Maxx®*, Flame®, Midas®*, OnDuty®*), imazapyr (Arsenal Xpress®*, Intervix®*, B Imidazolinones Lightning®*, Midas®*, OnDuty®*), imazethapyr (Lightning®*, Spinnaker®) B Pyrimidinylthiobenzoates bispyribac (Nominee®), pyrithiobac (Staple®) C Amides: propanil (Stam®) C Benzothiadiazinones: bentazone (Basagran®, -

Classification of Herbicides
Title of the course : Weed Management Credit: 3(2+1) Class : 3rd Year IInd Semester Title of the topic : Principles of weed management College : Krishi vigyan Kendra,College of Agriculture, Rewa, JNKVV, Jabalpur Name of Teacher : Dr. (Mrs.) Smita Singh Classification of Herbicides Herbicides: Chemical method of weed control is very effective in certain cases and have great scope provided the herbicides are cheap, efficient and easily available. The chemicals used for killing the weeds or inhibiting growth of weeds are called herbicides (Weedicides). Classification of Herbicides: Herbicides are classified in different ways: A) First Group Chemical Herbicides: I) Classification of herbicides according to chemical composition. II) Classification of herbicides according to their use. III) Classification of herbicides based on time of application. IV) Classification of herbicides according to Formulation. V) Classification of herbicides according to residual effect. B) Second Group – Bio herbicides C) Third Group herbicidal mixtures. Classification of herbicide I) Classification of Herbicide Based on Chemical Nature or Composition Compounds having chemical affinities are grouped together. This is useful in liting and characterising herbicides. i) Inorganic Herbicides:Contain no carbon actions in their molecules. These were the first chemicals used for weed control before the introduction of the organic compounds, example are: a) Acids:Arsenic acid, arsenious acid, arsenic trioxide sulphuric acid. b) Salts:Borax, copper sulphate, ammonium sulphate, Na chlorate , Na arsenite , copper nitrate. ii) Organic Herbicides:Oils and non oils contain carbon and hydrogen in their molecules. a) Oils: Diesel oil, standard solvent, xylene-type, aromatic oils, polycyclic , aromatic oils etc. b) Aliphatics:Dalapon, TCA, Acrolein, Glyphosphate methyl bromide. -

Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 Theinternational Programme on Chemical Safety (IPCS) Was Established in 1980
The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 cation Hazard of Pesticides by and Guidelines to Classi The WHO Recommended Classi The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 TheInternational Programme on Chemical Safety (IPCS) was established in 1980. The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. This publication was developed in the IOMC context. The contents do not necessarily reflect the views or stated policies of individual IOMC Participating Organizations. The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase international coordination in the field of chemical safety. The Participating Organizations are: FAO, ILO, UNDP, UNEP, UNIDO, UNITAR, WHO, World Bank and OECD. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment. WHO recommended classification of pesticides by hazard and guidelines to classification, 2019 edition ISBN 978-92-4-000566-2 (electronic version) ISBN 978-92-4-000567-9 (print version) ISSN 1684-1042 © World Health Organization 2020 Some rights reserved. -

5. Key Post-Emergent Modes of Action
5. KEY POST-EMERGENT MODES OF ACTION When new mode of action herbicides are first introduced, of this enzyme is reduction in the production of fatty acids manufacturers typically provide robust formulations and use required for construction of cell membranes needed for rates. There is often a high level of “forgiveness” in the label. new cell production. As resistant populations are selected over time, there is The ACCase enzyme in most broadleaf plants is insensitive sometimes a period where the herbicide may still be useful, to herbicides from this herbicide mode of action, and hence albeit with reduced performance. In these situations of low- there is acceptable crop tolerance in most broadleaf crops level or emerging resistance, it is critical that users seek to and no efficacy on most broadleaf weeds. Some exceptions maximise application conditions to ensure everything possible exist. For example, haloxyfop is able to control the broadleaf is done to enhance the herbicide performance. weed storksbill or geranium (Erodium spp.) while high rates of clethodim can damage canola, particularly when flowering. Understanding how each of the key modes of action available for post-emergent weed control work, how they The three sub-groups of Group A herbicides bind to the target enter and translocate in the plant, and what is required to enzyme at slightly different, and overlapping, amino acids. This differential binding can lead to differences in target site maximise efficacy is critical knowledge for maximising field herbicide resistance patterns both between and within the performance. sub groups (refer to the Acetyl CoA Carboxylase inhibitors This chapter coves the key modes of actions used for post- section under section 6.3.1.1. -

Registration Division Conventional Pesticides -Branch and Product
Registration Division Conventional Pesticides - Branch and Product Manager (PM) Assignments For a list of Branch contacts, please click the following link: http://www2.epa.gov/pesticide-contacts/contacts-office-pesticide-programs-registration-division Branch FB=Fungicide Branch. FHB=Fungicide Herbicide Branch. HB=Herbicide Branch. Abbreviations: IVB*= Invertebrate-Vertebrate Branch 1, 2 or 3. MUERB=Minor Use and Emergency Response Branch. Chemical Branch PM 1-Decanol FHB RM 20 1-Naphthaleneacetamide FHB RM 20 2, 4-D, Choline salt HB RM 23 2,4-D HB RM 23 2,4-D, 2-ethylhexyl ester HB RM 23 2,4-D, butoxyethyl ester HB RM 23 2,4-D, diethanolamine salt HB RM 23 2,4-D, dimethylamine salt HB RM 23 2,4-D, isopropyl ester HB RM 23 2,4-D, isopropylamine salt HB RM 23 2,4-D, sodium salt HB RM 23 2,4-D, triisopropanolamine salt HB RM 23 2,4-DB HB RM 23 2,4-DP HB RM 23 2,4-DP, diethanolamine salt HB RM 23 2,4-DP-p HB RM 23 2,4-DP-p, 2-ethylhexyl ester FB RM 21 2,4-DP-p, DMA salt HB RM 23 2-EEEBC FB RM 21 2-Phenylethyl propionate FHB RM 20 4-Aminopyridine IVB3 RM 07 4-Chlorophenoxyacetic acid FB RM 22 4-vinylcyclohexene diepoxide IVB3 RM 07 Abamectin IVB3 RM 07 Acephate IVB2 RM 10 Acequinocyl IVB3 RM 01 Acetaminophen IVB3 RM 07 Acetamiprid IVB3 RM 01 Acetic acid, (2,4-dichlorophenoxy)-, compd. with methanamine (1:1) HB RM 23 Acetic acid, trifluoro- FHB RM 20 Acetochlor HB RM 25 Acibenzolar-s-methyl FHB RM 24 Acid Blue 9 HB RM 23 Acid Yellow 23 HB RM 23 Sunday, June 06, 2021 Page 1 of 17 Chemical Branch PM Acifluorfen HB RM 23 Acrinathrin IVB1 RM 03 -

At Least 394 Pesticides May Affect Endangered and Threatened Species
At least 394 pesticides may affect endangered and threatened species: 1,3-Dichloropropene 10,10'-Oxybisphenoxyarsine 2-(2,4-DP), dimethylamine salt 2,4-D, 2-ethylhexyl ester 2,4-D, butoxyethanol ester 2,4-D, diethanolamine salt 2,4-D, diethylamine salt 2,4-D, Dimethylamine salt 2,4-D, isooctyl ester 2,4-D, isopropyl ester 2,4-D, isopropylamine salt 2,4-D, salts and esters 2,4-D, sodium salt 2,4-D, triisopropanolamine salt 2,4-DB acid 2,4-DB, dimethylamine salt 2,4-DP-P, Dimethylamine salt 2,4-DP-P, isooctyl ester 2,4-DP, isooctyl ester 2,4-DP,2-ethylhexyl ester 3-chloro-p-toluidine hydrochloride 3-iodo-2-propynyl butyl carbamate 3-Trifluoromethyl-4-nitrophenol 4-aminopyridine Acephate Acequinocyl Acetamiprid Acetochlor Acibenzolar-S-methyl Acrolein Alachlor Aldicarb Alpha-chlorohydrin Aluminum phosphide Ametryne Aminopyralid potassium salt Aminopyralid and salts Aminopyralid, triisopropanolamine salt Amitraz Amitrole Ammonium bromide Antimycin A Arsenic acid Arsenic pentoxide Arsenic trioxide Atrazine Avermectin Azinphos-methyl Azoxystrobin Benfluralin Bensulide Bentazon and salts Bentazon, sodium salt Beta-cyfluthrin Bethoxazin Bifenazate Bifenthrin Bis-(N-cyclohexyldiazeniumdioxy)-copper Brodifacoum Bromacil and salts Bromacil, lithium salt Bromadiolone Bromethalin Bromoxynil butyrate Bromoxynil heptanoate Bromoxynil octanoate Bromoxynil, salts and esters Buprofezin Butralin Butylate Captan Carbaryl Carbendazim Carbendazim phosphate Carbofuran Carboxin Chelerythrine chloride-sanguinarine chloride mixture Chlorantraniliprole Chlorethoxyphos -

Massachusetts Oil and Hazardous Material List
SUBPART P: MASSACHUSETTS OIL AND HAZARDOUS MATERIAL LIST TABLE OF CONTENTS TABLE 1 - MASSACHUSETTS OIL AND HAZARDOUS MATERIAL LIST (ALPHABETICAL LISTING) TABLE 2 - MASSACHUSETTS OIL AND HAZARDOUS MATERIAL LIST (BY CAS NUMBER ORDER) NOTES: The Massachusetts Oil and Hazardous Materials List (MOHML) contains oils and hazardous materials subject to 310 CMR 40.0000 and their reportable quantities (RQs) and reportable concentrations (RCs). These values are referred to in the notification requirements (310 CMR 40.0300). This list is provided both alphabetically in Table 1 and by Chemical Abstracts Service Number (CAS Number) in Table 2. The CAS number is a unique number assigned to a substance. Both tables identify other lists on which a substance appears by using name source codes. These codes are as follows: Name Source 1 - The Department of Transportation (DOT) Hazardous Materials List (49 CFR Part 172.101 Hazardous Materials Table) Name Source 2 - The Resource Conservation and Recovery Act Appendix VIII List (40 CFR Part 261 - Appendix VIII Hazardous Constituents) Name Source 3 - The Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) Hazardous Substance and Waste Stream Lists (40 CFR Part 302 - Table 302.4) Name Source 4 - The Extremely Hazardous Substance List as mandated by Superfund Amendments and Reauthorization Act, Title III, Section 302 (40 CFR Part 355 Appendices A and B) Name Source 5 - DEP Allowable Ambient Limits (AALs) and Drinking Water Guidelines Name Source 6 - The Massachusetts Substance List (MSL)(105 CMR 670.000: “Right to Know” Appendix A) Name Source 7 - The Chemical Abstracts name, 9th collective period, 1972-1976 Name Source 8 - The EPA Right to Know list, Section 313 of the Emergency Planning and Community Right to Know Act of 1986 (40 CFR Part 372.65). -
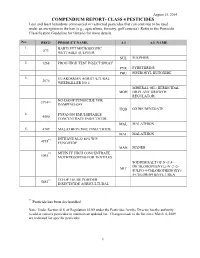
Class 4 Pesticides
August 15, 2014 COMPENDIUM REPORT- CLASS 4 PESTICIDES Less and least hazardous commercial or restricted pesticides that can continue to be used under an exception to the ban (e.g., agriculture, forestry, golf courses). Refer to the Pesticide Classification Guideline for Ontario for more details. No. REG# PRODUCT NAME A.I A.I. NAME 1. BARTLETT MICROSCOPIC 873 WETTABLE SULPHUR SUL SULPHUR 2. 1268 PRO® HIGH TEST INSECT SPRAY PYR PYRETHRINS PBU PIPERONYL BUTOXIDE 3. GUARDSMAN AGRICULTURAL 2076 WEEDKILLER NO.1 MINERAL OIL (HERBICIDAL MOH OR PLANT GROWTH REGULATOR) NO-DAMP FUNGICIDE FOR 3794** DAMPING-OFF HQB OXINE BENZOATE 4. FYFANON EMULSIFIABLE 4590 CONCENTRATE INSECTICIDE MAL MALATHION 5. 4709 MALATHION 500E INSECTICIDE MAL MALATHION DITHANE M-22 80% W.P. 4918 FUNGICIDE MAN MANEB MITIN FF HIGH CONCENTRATE ** 5095 MOTHPROOFING FOR TEXTILES SODIUM SALT OF N-(3,4- DICHLOROPHENYL)-N'-2 (2- MIT SULFO-4-CHLOROPHENOXY)- 5-CHLOROPHENYL UREA CO-OP LOUSE POWDER 5663** INSECTICIDE AGRICULTURAL ** Pesticide has been declassified Note: Under Section 4(1) of Regulation 63/09 under the Pesticides Act the Director has the authority to add or remove pesticides to maintain an updated list. Changes made to the list since March 4, 2009 are indicated for specific pesticides. 1 August 15, 2014 COMPENDIUM REPORT- CLASS 4 PESTICIDES No. REG# PRODUCT NAME A.I A.I. NAME ROT ROTENONE 6. MALATHION 500 EMULSIFIABLE 5821 CONCENTRATE INSECTICIDE MAL MALATHION 7. 5931 2,4-D AMINE 600 HERBICIDE 2,4-D PRESENT AS AMINE SALTS (DIMETHYLAMINE DXB SALT, DIETHANOLAMINE SALT, OR OTHER AMINE SALTS) TROPOTOX LIQUID SELECTIVE 5937** WEEDKILLER MCPB PRESENT AS SODIUM MBS SALT 8.