At Least 394 Pesticides May Affect Endangered and Threatened Species
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Restricted Use Product Summary Report
Page 1 of 17 Restricted Use Product Summary Report (January 19, 2016) Percent Active Registration # Name Company # Company Name Active Ingredient(s) Ingredient 4‐152 BONIDE ORCHARD MOUSE BAIT 4 BONIDE PRODUCTS, INC. 2 Zinc phosphide (Zn3P2) 70‐223 RIGO EXOTHERM TERMIL 70 VALUE GARDENS SUPPLY, LLC 20 Chlorothalonil 100‐497 AATREX 4L HERBICIDE 100 SYNGENTA CROP PROTECTION, LLC 42.6 Atrazine 100‐585 AATREX NINE‐O HERBICIDE 100 SYNGENTA CROP PROTECTION, LLC 88.2 Atrazine 100‐669 CURACRON 8E INSECTICIDE‐MITICIDE 100 SYNGENTA CROP PROTECTION, LLC 73 Profenofos 100‐817 BICEP II MAGNUM HERBICIDE 100 SYNGENTA CROP PROTECTION, LLC 33; 26.1 Atrazine; S‐Metolachlor 100‐827 BICEP LITE II MAGNUM HERBICIDE 100 SYNGENTA CROP PROTECTION, LLC 28.1; 35.8 Atrazine; S‐Metolachlor 100‐886 BICEP MAGNUM 100 SYNGENTA CROP PROTECTION, LLC 33.7; 26.1 Atrazine; S‐Metolachlor 100‐898 AGRI‐MEK 0.15 EC MITICIDE/INSECTICIDE 100 SYNGENTA CROP PROTECTION, LLC 2 Abamectin 100‐903 DENIM INSECTICIDE 100 SYNGENTA CROP PROTECTION, LLC 2.15 Emamectin benzoate 100‐904 PROCLAIM INSECTICIDE 100 SYNGENTA CROP PROTECTION, LLC 5 Emamectin benzoate 100‐998 KARATE 1EC 100 SYNGENTA CROP PROTECTION, LLC 13.1 lambda‐Cyhalothrin 100‐1075 FORCE 3G INSECTICIDE 100 SYNGENTA CROP PROTECTION, LLC 3 Tefluthrin Acetochlor; Carbamothioic acid, dipropyl‐ 100‐1083 DOUBLEPLAY SELECTIVE HERBICIDE 100 SYNGENTA CROP PROTECTION, LLC 16.9; 67.8 , S‐ethyl ester 100‐1086 KARATE EC‐W INSECTICIDE 100 SYNGENTA CROP PROTECTION, LLC 13.1 lambda‐Cyhalothrin 100‐1088 SCIMITAR GC INSECTICIDE 100 SYNGENTA CROP PROTECTION, -

Global Insecticide Use for Vector-Borne Disease Control
WHO/CDS/NTD/WHOPES/GCDPP/2007.2 GLOBAL INSECTICIDE USE FOR VECTOR-BORNE DISEASE CONTROL M. Zaim & P. Jambulingam DEPARTMENT OF CONTROL OF NEGLECTED TROPICAL DISEASES (NTD) WHO PESTICIDE EVALUATION SCHEME (WHOPES) First edition, 2002 Second edition, 2004 Third edition, 2007 © World Health Organization 2007 All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either express or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use. The named authors alone are responsible for the views expressed in this publication. CONTENTS Page Acknowledgements i Introduction 1 Collection of information 2 Data analysis and observations on reporting 3 All uses in vector control 6 Malaria vector control 22 Dengue vector control 38 Chagas disease vector control 48 Leishmaniasis vector control 52 Other vector-borne disease control 56 Selected insecticides – DDT 58 Selected insecticides – Insect growth regulators 60 Selected insecticides – Bacterial larvicides 62 Country examples 64 Annex 1. -
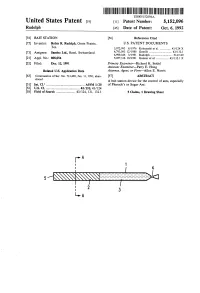
United States Patent (19) 11 Patent Number: 5,152,096 Rudolph 45 Date of Patent: Oct
III USOO5152096A United States Patent (19) 11 Patent Number: 5,152,096 Rudolph 45 Date of Patent: Oct. 6, 1992 (54) BAIT STATION (56) References Cited (75) Inventor: Robin R. Rudolph, Grain Prairie, U.S. PATENT DOCUMENTS Tex. 3,972,993 8/1976 Kobayashi et al................ 43/124 X 4,793,093 12/1988 Gentile ............................... 43/132.1 (73) Assignee: Sandoz Ltd., Basel, Switzerland 4,999,346 3/1991 Rudolph .............................. 514/120 21 Appl. No.: 808,054 5,057,316 10/1991 Gunner et al. ................. 43/132.1 X 22 Filed: Dec. 12, 1991 Primary Examiner-Richard K. Seidel Assistant Examiner-Patty E. Hong Related U.S. Application Data Attorney, Agent, or Firm-Allen E. Norris 63 Continuation of Ser. No. 713,480, Jun. 11, 1991, aban 57) ABSTRACT doned. A bait station device for the control of ants, especially (51) Int. Cl. ............................................... A0M 1/20 of Pharaoh's or Sugar Ant. (52 U.S. C. ......................................... 43/131; 43/124 58) Field of Search ....................... 43/124, 131, 132.1 5 Claims, 1 Drawing Sheet U.S. Patent Oct. 6, 1992 5,152,096 DSN a- 5,152,096 1. 2 enting the ants with a combination of an insect growth BAIT STATION regulant (IGR) bait and insecticide bait in such a way that the worker ants have to forage their way through This is a continuation of application Ser. No. the IGR bait to reach the insecticide bait. 07/713,480, filed Jun. 11, 1991, now abandoned. 5 In this way foraging worker ants will transport back The present invention concerns a bait station device to nests for feeding of the colony IGR bait and upon for the control of ants, especially of Pharaoh's or Sugar exhausting the available IGR bait will themselves ingest Ant. -
MN2000 FSAC 006 Revised1978.Pdf
-i .., ,,, (I-,~-- -' d ~-- C:::- ,, AG RIC UL TURAL EXTENSION SERVICE Afr{ ,<"·<,'>· , -> ',' "\, '. '' ~'¥) AGRICULTURAL CHEMICALS FACT SHEET No. 6-1978 Chemicals for'Weed Cop: GERALD R. MILLER . \~:::sr" This fact sheet is intended only as a summary of suggested Herbicide names and formulations alternative chemicals for weed control in corn. Label informa Common Trade Concentration and tion should be read and followed exactly. For further informa name name commercial formulation 1 tion, see Extension Bulletin 400, Cultural and Chemical Weed Control in Field Crops. Alachlor Lasso 4 lb/gal L Lasso 11 15% G Selection of an effective chemical or combination of chemicals Atrazine AAtrex, 80% WP, 4 lb/gal L should be based on consideration of the following factors: others 80%WP -Clearance status of the chemical Atrazine and propachlor AAtram 20% G -Use of the crop Bentazon Basagran 4 lb/gal L -Potential for soil residues that may affect following crops Butylate and protectant Sutan+ 6.7 lb/gal L -Kinds of weeds Cyanazine Bladex 80% WP, 15% G, 4 lb/gal L -Soil texture Dicamba Banvel 4 lb/gal L -pH of soil Dicamba and 2,4-D Banvel-K 1.25 lb/gal dicamba -Amount of organic matter in the soil 2.50 lb/gal 2,4-D -Formulation of the chemical EPTC and protectant Eradicane 6.7 lb/gal L -Application equipment available Linuron Lorox 50%WP -Potential for drift problems Metolachlor Dual 6 lb/gal L Propachlor Bexton, 65% WP, 20% G, 4 lb/gal L Ramrod 2,4-D Several Various 1 G = granular, L = liquid, WP= wettable powder Effectiveness of herbicides on -

(12) United States Patent (10) Patent No.: US 8,586,504 B2 Wright Et Al
USOO85865.04B2 (12) United States Patent (10) Patent No.: US 8,586,504 B2 Wright et al. (45) Date of Patent: Nov. 19, 2013 (54) HERBICIDAL COMPOSITIONS CONTAINING FOREIGN PATENT DOCUMENTS GLYPHOSATE AND A PYRONE ANALOG AU 100.73/92 B 10, 1992 CA 2340240 A1 2, 2000 (75) Inventors: Daniel R. Wright, St. Louis, MO (US); EP O 808 569 A1 11, 1997 Joseph J. Sandbrink, Chesterfield, MO GB 2267 825 A 12/1993 (US); Paul G. Ratliff, Olivette, MO WO 99/00013 1, 1999 WO OO,30452 6, 2000 (US) WO OO,642.57 11, 2000 WO OO/67571 11, 2000 (73) Assignee: Monsanto Technology LLC, St. Louis, WO O1/35740 A2 5, 2001 MO (US) WO 02/21924 A2 3, 2002 (*) Notice: Subject to any disclaimer, the term of this OTHER PUBLICATIONS patent is extended or adjusted under 35 Exhibit PMH-17 Supplemental Labeling regarding Roundup Pro U.S.C. 154(b) by 0 days. Herbicide by Monsanto, EPA Reg. No. 524-475 (Nov. 1995), 11 pageS. (21) Appl. No.: 13/404,861 Exhibit PMH-18 Notice of Pesticide Registration issued on Oct. 5, 2000, 35 pages. Exhibit PMH-19 EPA Application for Pesticide, ID No. 200405 (22) Filed: Feb. 24, 2012 (Sep. 1995), 33 pages. Exhibit PMH-20-Documentation regarding Starmas Racun/ (65) Prior Publication Data Rumpai Herbicide (bears the year 2003), 10 pages. Exhibit PMH-21—Documentation regarding Starmix Racun/ US 2012/O157309 A1 Jun. 21, 2012 Rumpai Herbicide (Date Unknown), 3 pages. Exhibit PMH-22—article entitled Control of Eucalyptus grandis cut stumps by Keith Little et al., ICFR Bulletin Series, No. -

2,4-Dichlorophenoxyacetic Acid
2,4-Dichlorophenoxyacetic acid 2,4-Dichlorophenoxyacetic acid IUPAC (2,4-dichlorophenoxy)acetic acid name 2,4-D Other hedonal names trinoxol Identifiers CAS [94-75-7] number SMILES OC(COC1=CC=C(Cl)C=C1Cl)=O ChemSpider 1441 ID Properties Molecular C H Cl O formula 8 6 2 3 Molar mass 221.04 g mol−1 Appearance white to yellow powder Melting point 140.5 °C (413.5 K) Boiling 160 °C (0.4 mm Hg) point Solubility in 900 mg/L (25 °C) water Related compounds Related 2,4,5-T, Dichlorprop compounds Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) 2,4-Dichlorophenoxyacetic acid (2,4-D) is a common systemic herbicide used in the control of broadleaf weeds. It is the most widely used herbicide in the world, and the third most commonly used in North America.[1] 2,4-D is also an important synthetic auxin, often used in laboratories for plant research and as a supplement in plant cell culture media such as MS medium. History 2,4-D was developed during World War II by a British team at Rothamsted Experimental Station, under the leadership of Judah Hirsch Quastel, aiming to increase crop yields for a nation at war.[citation needed] When it was commercially released in 1946, it became the first successful selective herbicide and allowed for greatly enhanced weed control in wheat, maize (corn), rice, and similar cereal grass crop, because it only kills dicots, leaving behind monocots. Mechanism of herbicide action 2,4-D is a synthetic auxin, which is a class of plant growth regulators. -

Herbicide Mode of Action Table High Resistance Risk
Herbicide Mode of Action Table High resistance risk Chemical family Active constituent (first registered trade name) GROUP 1 Inhibition of acetyl co-enzyme A carboxylase (ACC’ase inhibitors) clodinafop (Topik®), cyhalofop (Agixa®*, Barnstorm®), diclofop (Cheetah® Gold* Decision®*, Hoegrass®), Aryloxyphenoxy- fenoxaprop (Cheetah®, Gold*, Wildcat®), fluazifop propionates (FOPs) (Fusilade®), haloxyfop (Verdict®), propaquizafop (Shogun®), quizalofop (Targa®) Cyclohexanediones (DIMs) butroxydim (Factor®*), clethodim (Select®), profoxydim (Aura®), sethoxydim (Cheetah® Gold*, Decision®*), tralkoxydim (Achieve®) Phenylpyrazoles (DENs) pinoxaden (Axial®) GROUP 2 Inhibition of acetolactate synthase (ALS inhibitors), acetohydroxyacid synthase (AHAS) Imidazolinones (IMIs) imazamox (Intervix®*, Raptor®), imazapic (Bobcat I-Maxx®*, Flame®, Midas®*, OnDuty®*), imazapyr (Arsenal Xpress®*, Intervix®*, Lightning®*, Midas®* OnDuty®*), imazethapyr (Lightning®*, Spinnaker®) Pyrimidinyl–thio- bispyribac (Nominee®), pyrithiobac (Staple®) benzoates Sulfonylureas (SUs) azimsulfuron (Gulliver®), bensulfuron (Londax®), chlorsulfuron (Glean®), ethoxysulfuron (Hero®), foramsulfuron (Tribute®), halosulfuron (Sempra®), iodosulfuron (Hussar®), mesosulfuron (Atlantis®), metsulfuron (Ally®, Harmony®* M, Stinger®*, Trounce®*, Ultimate Brushweed®* Herbicide), prosulfuron (Casper®*), rimsulfuron (Titus®), sulfometuron (Oust®, Eucmix Pre Plant®*, Trimac Plus®*), sulfosulfuron (Monza®), thifensulfuron (Harmony®* M), triasulfuron (Logran®, Logran® B-Power®*), tribenuron (Express®), -

40 CFR Ch. I (7–1–18 Edition) § 455.61
§ 455.61 40 CFR Ch. I (7–1–18 Edition) from: the operation of employee show- § 455.64 Effluent limitations guidelines ers and laundry facilities; the testing representing the degree of effluent of fire protection equipment; the test- reduction attainable by the applica- ing and emergency operation of safety tion of the best available tech- showers and eye washes; or storm nology economically achievable water. (BAT). (d) The provisions of this subpart do Except as provided in 40 CFR 125.30 not apply to wastewater discharges through 125.32, any existing point from the repackaging of microorga- source subject to this subpart must nisms or Group 1 Mixtures, as defined achieve effluent limitations rep- under § 455.10, or non-agricultural pes- resenting the degree of effluent reduc- ticide products. tion attainable by the application of the best available technology economi- § 455.61 Special definitions. cally achievable: There shall be no dis- Process wastewater, for this subpart, charge of process wastewater pollut- means all wastewater except for sani- ants. tary water and those wastewaters ex- § 455.65 New source performance cluded from the applicability of the standards (NSPS). rule in § 455.60. Any new source subject to this sub- § 455.62 Effluent limitations guidelines part which discharges process waste- representing the degree of effluent water pollutants must meet the fol- reduction attainable by the applica- lowing standards: There shall be no dis- tion of the best practicable pollut- charge of process wastewater pollut- ant control technology (BPT). ants. Except as provided in 40 CFR 125.30 through 125.32, any existing point § 455.66 Pretreatment standards for existing sources (PSES). -

Reduction of Nitroaromatic Pesticides with Zero-Valent Iron
Chemosphere 54 (2004) 255–263 www.elsevier.com/locate/chemosphere Reduction of nitroaromatic pesticides with zero-valent iron Young-Soo Keum, Qing X. Li * Department of Molecular Biosciences and Bioengineering, University of Hawaii, 1955 East-West Road, Ag Sci 218, Honolulu, HI 96822, USA Received 5 February 2003; received in revised form 4 June 2003; accepted 4 August 2003 Abstract Reduction of eleven nitroaromatic pesticides was studied with zero-valent iron powder. Average half-lives ranged from 2.8 to 6.3 h and the parent compounds were completely reduced after 48–96 h. The di-nitro groups of the 2,6- dinitroaniline herbicides were rapidly reduced to the corresponding diamines, with a negligible amount of partially reduced monoamino or nitroso products. Low levels of de-alkylated products were observed after 10 days. The nitro group of the organophosphorus insecticides was reduced dominantly to the monoamines but in a slower rate than the 2,6-dinitroanilines. A trace amount of oxon products was found. Reduction of nitro to amino was also the predominant reaction for the diphenyl ether herbicides. Aromatic de-chlorination and de-alkylation were minor reactions. These amine products were more stable than the parent compounds and 60% or more of the amines were detected after two weeks. Humic acid decreased the reduction rates of pendimethalin, and dichlone (a known quinone redox mediator) counteracted the effect of humic acid on the reactivity. Storage of iron powder under air decreased the reactivity very rapidly due to iron oxidation. Repeated use of iron powder also showed similar results. The reduced activity of air- oxidized iron was recovered by purging with hydrogen, but not nitrogen. -

FSC-TPL-01-002 Application for a Derogation to Use a Highly Hazardous Pesticide
FSC-TPL-01-002 Application for a derogation to use a highly hazardous pesticide. 2,4-D, 2-ethylhexyl ester Name and contact details of SCS certification body requesting Dave Wager derogation: [email protected] 510 251-7049 To be completed at the national level Active ingredient for which derogation requested: Geographical scope of All states of USA requested derogation: Is there an accredited or preliminarily accredited FSC FSC US standard Forest Stewardship Standard applicable to the territory concerned? Requested time period for derogation: 5 years (nb Derogations shall normally be issued for a five-year period. There will be a presumption against renewal of a derogation after the expiry of the five-year period). 1. Demonstrated need Need may be demonstrated where: - The pesticide is used for protecting native species and forests against damage caused by introduced species or for protecting human health against dangerous diseases, OR - Use of the pesticide is obligatory under national laws or regulations, OR - Use of the pesticide is the only economically, environmentally, socially and technically feasible way of controlling specific organisms which are causing severe damage in natural forests or plantations in the specified country (as indicated by consideration, assessments and preferably field-trials of alternative non- chemical or less toxic pest-management methods) Explain how the proposed use complies with the specified criteria for need, including the consideration of alternatives which do not require the use of pesticides on the FSC list of ‘highly hazardous pesticides’: Overview 2,4-D ester is a selective herbicide used to control broad leaved plants such as woody species and forbs. -

PUBLIC WATER SUPPLY SAMPLING PLAN for Contaminants with a Vermont Health Advisory – May 2020
PROPOSED PUBLIC WATER SUPPLY SAMPLING PLAN For Contaminants with a Vermont Health Advisory – May 2020 Vermont Department of Environmental Conservation Drinking Water & Groundwater Protection Division A Plan to Sample for Chemicals with a Vermont Health Advisory As required by Act 21 (2019), Section 10(b), the Secretary of the Agency of Natural Resources, on or before January 1, 2020, must publish for public review and comment a plan to collect data for contaminants in drinking water from public community water systems and all non-transient non-community water systems, for which a health advisory has been established, but no Maximum Contaminant Level has been adopted. These health advisories are referred to as Vermont Health Advisories (VHAs) in this document. 1 | P a g e TABLE OF CONTENTS I. Executive Summary …………………………………………………………………………………………..Page 3 II. Background ……………………………………………………………………………………………………… Page 4 III. Determining the VHA contaminants for sampling at public water systems ………..Page 6 IV. Sampling Considerations …..…………………………………………………………………………….. Page 10 V. Proposed Sampling Plan ………………………………………………………………………….………..Page 12 Attachments Table 1 Complete List of Vermont Health Advisories (VHAs) …………………………………………..Page 13 Table 2 Proposed List of VHAs with Potential Concern ……………………………………………………Page 18 2 | P a g e I. Executive Summary The Secretary of the Agency of Natural Resources was tasked with developing a sampling plan for public review, for certain drinking water contaminants that have an established health advisory, also known as the Vermont Health Advisory (VHA) but have no Maximum Contaminant Level (MCL). This Sampling Plan (Plan) is targeted to public community and public non- transient non-community water systems. To provide context for public water system regulation, and standards that apply, a discussion of how VHAs and MCLs are determined is given. -

Special Report 354 April 1972 Agricultural Experiment Station
ORTMAL DO ;10T REMOVE 7.9 m FILE Special Report 354 April 1972 Agricultural Experiment Station Oregon State University, Corvallis I FIELD APPLICATION OF HERBICIDES--AVOIDING DANGER TO FISH Erland T. Juntunen Department of Fisheries and Wildlife Oregon State University Corvallis, Oregon and Logan A. Norris Pacific Northwest Forestry Sciences Laboratory and Range Experiment Station Forest Service, U. S. Department of Agriculture Corvallis, Oregon April, 1972 Trade names are used in this publication solely to provide specific information. No endorsement of products is intended nor is criticism implieLl to products mentioned or omitted. Recommendations are not made concerning safe use of products nor is any guarantee or warranty of results or effects of the products intended or implied. ii Chemical weed and brush control with herbicides is an important land management practice in modern agriculture and forestry. In some cases, herbicides are applied directly to bodies of water for aquatic weed control. More commonly, herbicides are applied to lands adjacent to waterways for general weed and brush control. The responsible applicator will avoid damage to fishery resources by being fully aware of a particular herbicides potential hazard to fish. Herbicide applications should be considered hazardous to fish when there is the probability fish will be exposed to herbicide concen- trations which are harmful. This bulletin offers information that will aid in selecting the particular herbicides and formulations of least hazard to fish considering the toxicity of the herbicide and the poten- tial for its entry into streams, lakes, or ponds. Entry of Herbicides into the Aquatic Environment In aquatic weed control, the effective concentration of herbicide in the water depends on the rate of application, the rate of the spread of the chemical, the size and chemical composition of the body of water, the rate of degradation or adsorption of the chemical on sediments, and the rate of mixing of treated water with untreated water.