J&J: It Will Take More Than a Band-Aid
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Women in Business Awards Luncheon at the Hotel Irvine, Where Aston Martin Americas President Laura Schwab Delivered the Keynote Address
10.5.20 SR_WIB.qxp_Layout 1 10/2/20 12:14 PM Page 29 WOMEN IN BUSINESS NOMINEES START ON PAGE B-60 INSIDE 2019 WINNERS GO BIG IN IRVINE, LAND NEW PARTNERS, INVESTMENTS PAGE 30 PRESENTED BY DIAMOND SPONSOR PLATINUM SPONSORS GOLD SPONSOR SILVER SPONSORS 10.5.20 SR_WIB.qxp_Layout 1 10/2/20 1:36 PM Page 30 30 ORANGE COUNTY BUSINESS JOURNAL www.ocbj.com OCTOBER 5, 2020 Winning Execs Don’t Rest on Their Laurels $1B Cancer Center Underway; Military Wins; Spanish Drug Investment Orange County’s business community last year celebrated the Business Journal’s 25th annual Women in Business Awards luncheon at the Hotel Irvine, where Aston Martin Americas President Laura Schwab delivered the keynote address. The winners, selected from 200 nominees, have not been resting on their laurels, even in the era of the coronavirus. Here are updates on what the five winners have been doing. —Peter J. Brennan Avatar Partners City of Hope Shortly after Marlo Brooke won the Busi- (AR) quality assurance solution for the U.S. As president of the City of Hope Orange employees down from Duarte. Area univer- ness Journal’s award for co-founding Hunt- Navy for aircraft wiring maintenance for the County, Annette Walker is orchestrating a sities could partner with City of Hope. ington Beach-based Avatar Partners Inc., Naval Air Systems Command’s Boeing V- $1 billion project to build one of the biggest, While the larger campus near the Orange she was accepted into the Forbes Technol- 22 Osprey aircraft. and scientifically advanced, cancer research County Great Park is being built, Walker in ogy Council, an invitation-only community Then the Air Force is using Avatar’s solu- centers in the world. -

Drug Information Center Highlights of FDA Activities
Drug Information Center Highlights of FDA Activities – 9/1/20 – 9/30/20 FDA Drug Safety Communications & Drug Information Updates: Efficacy & Safety Concerns for Atezolizumab in Combination with Paclitaxel 9/8/20 The FDA alerted health care professionals and patients that a clinical trial evaluating atezolizumab plus paclitaxel in patients with previously untreated inoperable locally advanced or metastatic triple negative breast cancer that the drug combination was not effective. The combination of atezolizumab with another paclitaxel formulation, paclitaxel protein‐bound, is currently approved for use in adult patients with metastatic triple negative breast cancer, but this continued approval may be contingent on the results of additional studies. Paclitaxel should NOT be used as a replacement for paclitaxel protein bound in clinical practice. Electronic Expanded Access Requests 9/23/20 The FDA announced that the Reagan‐Udall Foundation has launched Expanded Access eRequest, a tool to submit expanded access requests for individual patient expanded access for drugs and biologics in non‐emergency settings. The tool allows auto population of forms, uploading of relevant documents, links to resources for physicians, patients, and caregivers, and secure application submission to the FDA. Benzodiazepine Drug Class: Drug Safety Communication ‐ Boxed Warning Update 9/23/20 The FDA is requiring the Boxed Warning be updated for all benzodiazepines to address serious risks of abuse, addiction, physical dependence, and withdrawal across the medication class. Changes are also being incorporated in the Medication Guides, and other sections of the prescribing information including the Warnings and Precautions, Drug Abuse and Dependence, and Patient Counseling Information sections. Diphenhydramine (Benadryl): Serious Problems with High Doses 9/24/20 The FDA issued a warning that taking higher than recommended doses of the common OTC allergy medication diphenhydramine (Benadryl) can lead to serious heart problems, seizures, coma, or even death. -

Mcneil Consumer : Mdl No
IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF PENNSYLVANIA IN RE: MCNEIL CONSUMER : MDL NO. 2190 HEALTHCARE, ET AL., MARKETING : AND SALES PRACTICES LITIGATION : : Applies to: : ALL ACTIONS : MEMORANDUM McLaughlin, J. July 13, 2012 This multidistrict litigation arises out of quality control problems at the defendants’ facility manufacturing over- the-counter healthcare products in Fort Washington, Pennsylvania, which led to a series of recalls of those products. The named plaintiffs assert claims for economic loss on behalf of a putative nationwide class against Johnson & Johnson (“J&J”), McNeil Consumer Healthcare (“McNeil”), and four of their executives. The plaintiffs allege that they overpaid for the defendants’ products as a result of the recalls and the defendants’ scheme to conceal or downplay the scope of the quality control problems. The defendants, who have offered a coupon or cash refund to consumers who purchased recalled drugs, have moved to dismiss the operative complaint, and assert that the named plaintiffs lack constitutional standing and have not met the applicable pleading standard. The Court will grant the defendants’ motion because the plaintiffs have not pled facts that show a cognizable injury in fact, which is required to confer Article III standing. I. Procedural Background This litigation resulted from the consolidation of ten individual actions filed around the country. Haviland v. McNeil Consumer Healthcare, No. 10-2195, was filed in this Court on May 12, 2010, asserting economic injuries arising out of the April 30, 2010 recall of over-the-counter children’s drugs by McNeil, a part of the J&J “Family of Companies.” Eight additional cases, also arising out of the April 2010 recall, were filed in district courts around the country.1 All cases asserted claims for economic injury only, with the exception of Rivera v. -

Approved Prenatal Medications Pain Medications • Tylenol
Approved Prenatal Medications Pain Medications Tylenol (acetaminophen) for minor aches and pains, headaches. (Do not use: Aspirin, Motrin, Advil, Aleve, Ibuprofen.) Coughs/Colds Robitussin (Cough) Robitussin DM (non-productive cough) DO NOT USE TILL OVER 12 WEEKS Secrets and Vicks Throat Lozenges Mucinex Sore Throat Chloraseptic spray Saline Gargle Sucrets and Vicks Throat Lozenges Antihistamines/Allergies Zyrtec Claritin Benadryl Dimetapp Insomnia Benadryl Unison Hemorrhoids Preparation H Tucks Anusol Diarrhea Imodium (1-2 doses- if it persists please notify the office) BRAT diet (bananas, rice, applesauce, toast) Lice RID (only!) DO NOT USE Kwell Itching Benadryl Calamine or Caladryl Lotion Hydrocortisone cream Heartburn, Indigestion, Gas Tums Gas-X Mylanta Pepcid Maalox Zantac *DO NOT USE PEPTO BISMOL- it contains aspirin Decongestants Sudafed Robitussin CF- Only if over 12 weeks Tavist D Ocean Mist Nasal Spray (saline solutions) Nausea Small Frequent Meals Ginger Ale Vitamin B6 Sea Bands Yeast Infections Monistat Mycolog Gyne-lotrimin Toothache Orajel May see dentists, have cavity filled using Novocain or lidocaine, have x-rays with double lead shield, may have antibiotics in the Penicillin family (penicillin, amoxicillin) Sweetners- all should be consumed in moderation with water being consumed more frequently Nutrisweet (aspartame) Equal (aspartame) Splenda (sucralose) Sweet’n Low (saccharin) *note avoid aspartame if you have phenylketonuria (PKU) Constipation Colace Fibercon Citrucel Senokot Metamucil Milk of Magnesia Fiberall Miralax Eczema Hydrocortisone Cream Medications to AVOID Accurate Lithium Paxil Ciprofloxacin Tetracycline Coumadin Other Chemicals to AVOID Cigarettes Alcohol Recreational Drugs: marijuana, cocaine, ecstasy, heroin . -

Over-The-Counter (OTC) Medications Applies To: Tufts Health Ritogether and Tufts Health Together*
Over-the-Counter (OTC) Medications Applies to: Tufts Health RITogether and Tufts Health Together* As communicated in the November 1, 2018 Provider Update, the following changes are effective for fill dates on or after January 1, 2019. As a result of this change, some OTC medications will require prior authorization in certain circumstances as outlined below: Brand-Name OTC Medication Has a Covered Interchangeable Generic Version Available Afrin No Drip Advil capsule Advil tablet Advil PM tablet Afrin Nasal Spray Original nasal solution Afrin No Drip Aveeno Oatmeal Severe nasal Aleve tablet Baciguent ointment Benadryl capsule Bath Pak Treatment solution Benadryl Allergy Benadryl Allergy Benadryl Allergy Benadryl Extra Benefiber powder tablet capsule Liquid Strength cream Caltrate 600 +D Centrum Silver Betadine Swabstick Caltrate + D tablet Centrum liquid Plus Minerals tablet tablet Centrum Silver Centrum Ultra Men’s Children’s Advil Centrum tablet Cheracol-D syrup Adult 50+ tablet tablet suspension Children’s Benadryl Citracal Calcium + Children’s Benadryl Children’s Tylenol Chlor-Trimeton Allergy chewable D Slow Release Allergy liquid suspension syrup tablet tablet Citrucel Fiber Claritin-D 12 hour Claritin-D 24 hour Clear Cough Liquid Citrucel tablet Laxative powder tablet tablet PM Dimetapp DM Dex4 Fast Acting Dimetapp Cold and Colace capsule Conceptrol 4% gel Cough and Cold Glucose liquid Allergy elixir elixir Dristan nasal spray Dulcolax tablet D-Vi-Sol liquid Ecotrin tablet Evac powder Ex-Lax chewable Gas-X chewable Feosol tablet -

Johnson & Johnson Reports 2010 Second-Quarter Results
Johnson & Johnson Reports 2010 Second-Quarter Results: Sales of $15.3 Billion Increased 0.6% Versus 2009 Second-Quarter; EPS was $1.23 Excluding Special Items, 2010 Second-Quarter EPS was $1.21, an increase of 5.2%* NEW BRUNSWICK, N.J., July 20, 2010 /PRNewswire via COMTEX News Network/ -- Johnson & Johnson (NYSE: JNJ) today announced sales of $15.3 billion for the second quarter of 2010, an increase of 0.6% as compared to the second quarter of 2009. Operational results increased 0.1% and the positive impact of currency was 0.5%. Domestic sales declined 2.8%, while international sales increased 4.1%, reflecting operational growth of 3.0% and a positive currency impact of 1.1%. Net earnings and diluted earnings per share for the second quarter of 2010 were $3.4 billion and $1.23, respectively. Second- quarter 2010 net earnings included an after-tax gain of $67 million representing the net impact of litigation matters. Excluding this special item, net earnings for the current quarter were $3.4 billion and diluted earnings per share were $1.21, representing increases of 5.4% and 5.2%, respectively, as compared to the same period in 2009.* The Company updated its earnings guidance for full-year 2010 to $4.65 - $4.75 per share, which excludes the impact of special items. The Company's guidance now reflects the impact of the voluntary recalls announced earlier this year of certain over-the-counter medicines and the suspension of manufacturing at the McNeil Consumer Healthcare facility in Fort Washington, Pa., as well as unfavorable changes in foreign currency exchange rates. -
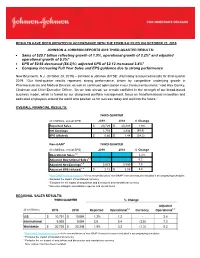
Sales of $20.7 Billion Reflecting Growth of 1.9%, Operational Growth of 3.2
RESULTS HAVE BEEN UPDATED IN ACCORDANCE WITH THE FORM 8-K FILED ON OCTOBER 23, 2019 JOHNSON & JOHNSON REPORTS 2019 THIRD-QUARTER RESULTS: • Sales of $20.7 billion reflecting growth of 1.9%, operational growth of 3.2%* and adjusted operational growth of 5.2%* • EPS of $0.66 decreased (54.2)%; adjusted EPS of $2.12 increased 3.4%* • Company increasing Full Year Sales and EPS guidance due to strong performance New Brunswick, N.J. (October 23, 2019) – Johnson & Johnson (NYSE: JNJ) today announced results for third-quarter 2019. “Our third-quarter results represent strong performance, driven by competitive underlying growth in Pharmaceuticals and Medical Devices, as well as continued optimization in our Consumer business,” said Alex Gorsky, Chairman and Chief Executive Officer. “As we look ahead, we remain confident in the strength of our broad-based business model, which is fueled by our disciplined portfolio management, focus on transformational innovation and dedicated employees around the world who position us for success today and well into the future.” OVERALL FINANCIAL RESULTS: THIRD QUARTER ($ in Millions, except EPS) 2019 2018 % Change Reported Sales $ 20,729 $ 20,348 1.9% Net Earnings 1,753 3,934 (55.4) EPS (diluted) $ 0.66 $ 1.44 (54.2) Non-GAAP* THIRD QUARTER ($ in Millions, except EPS) 2019 2018 % Change Operational Sales1,2 3.2% Adjusted Operational Sales1,3 5.2 Adjusted Net Earnings1,4 5,672 5,590 1.5 Adjusted EPS (diluted)1,4 $ 2.12 $ 2.05 3.4 1 Non-GAAP financial measure; refer to reconciliations of non-GAAP financial measures -

Benadryl (Diphenhydramine) Dosing Chart
1084 Cromwell Avenue Rocky Hill, CT 06067 (860) 721-7561 – Phone (860) 721-9199 – Fax Benadryl (Diphenhydramine) Dosing Chart *Ages 6 months & above* Dose every 6 hours: Dose for hives and/or itching. In general, once hives have started, you should continue regular dosing until there have not been hives for the entire day. Benadryl (Diphenhydramine) Liquid: Chewable Tablets: Tablets: Weight: 12.5mg/5ml 12.5mg each 25 mg each 17-21 pounds ¾ tsp. (3.75ml) Use Liquid Use Liquid Use 22-32 pounds 1 tsp. (5ml) 1 tablet Liquid or Chews Use 33-42 pounds 1 ½ tsp. (7.5ml) 1 ½ tablets Liquid or Chews 43-53 pounds 2 tsp. (10ml) 2 tablets 1 tablet 54-64 pounds 2 ½ tsp. (12.5ml) 2 ½ tablets 1 tablet 65-75 pounds 3 tsp. (15ml) 3 tablets 1 tablet 76-86 pounds 3 ½ tsp. (17.5ml) 3 ½ tablets 1 tablet >86 pounds 4 tsp. (20ml) 4 tablets 2 tablets EDUCATION ON CALL Administering Medicine Safely When your child isn’t feeling well, you want to relieve their discomfort as quickly as possible. Be prepared with information that can help you understand the differences between pediatric pain relievers and fever reducers, and how to administer them safely. Always read the label 1. Active ingredient: Ingredient that makes the medicine work 2. Uses: Symptoms the medicine treats 3. Directions: The amount of medicine to give and how often Know the difference TYLENOL® MOTRIN® Active ingredient: Acetaminophen Active ingredient: Ibuprofen • Treats pain & fever • Treats pain & fever • Gentle on tummies • Lasts up to 8 hours • Dosing available from your pediatrician for • Can be used for children 6 months of age or older children 6 months and younger Never give aspirin to children. -

Department of Public Health 105 Cmr 720.000
105 CMR: DEPARTMENT OF PUBLIC HEALTH 105 CMR 720.000: LIST OF INTERCHANGEABLE DRUG PRODUCTS Section 720.001: Purpose 720.002: Citation 720.010: Scope and Application 720.020: Definitions Standards 720:040: Commission Review of Relevant Drug Products 720.050: List of Interchangeable Drug Products 720.060: Drug Products Excluded 720.070: Amendments to the Massachusetts List of Interchangeable Drugs Procedures for Amending List of Interchangeable Drug Products 720.080: Procedures for Amending the Massachusetts List of Interchangeable Drugs 720.081: Petition to Amend List of Interchangeable Drug Products 720.082: Commission Review of Petition 720.083: Notice of Public Comment Period 720.084: Commission Recommendation of Amendments to Department 720.090: Department Adoption of Amendments 720.100: Severability 720.200: Appendix A: List of Interchangeable Drugs 720.001: Purpose The purpose of 105 CMR 720.000 is to establish a drug formulary, or list of interchangeable drug products, for use by physicians, other practitioners, and pharmacists licensed to practice within the commonwealth, so that consumers of prescription drug products may realize cost savings by buying less expensive, safe drug products. 720.002: Citation 105 CMR 720.000 shall be known as the 105 CMR 720.000: Massachusetts List of Interchangeable Drug Products. 720.010: Scope and Application 105 CMR 720.000 establishes the list of interchangeable drug products from which a pharmacist must interchange a reasonably available less expensive drug product than that written, when a prescription written by a practitioner indicates "interchange". 105 CMR 720.000 also establishes criteria and procedures for inclusion of drug products on this list. -

2020 Health for Humanity Report
2020 Health for Humanity Report Progress in Sustainability Contents | Message from Our CEO | Our Approach | United in Defeating COVID-19 | Better Health for All | Responsible Business Practices | Reporting Hub Contents Message from Our Chairman and CEO 3 2020 Year in Brief 4 Our Recognitions 5 Our Approach 6 Health for Humanity Strategy & Goals 9 Sustainability Governance 13 Sustainability Priorities 14 United in Defeating COVID-19 15 Caring for Patients 17 Supporting the Front Lines of Care 24 Protecting Employees 25 Supply Chain Resilience 28 Better Health for All 30 Innovation 31 Global Public Health Strategy 35 Access & Affordability 45 Strengthening Health Systems 48 Responsible Business Practices 52 Ethics & Values 53 Our People 60 Product Quality & Safety 74 Environmental Health 79 Responsible Supply Base 90 ABOVE: During the COVID-19 pandemic, Report overview community healthcare workers in Peru Reporting Hub 98 This Report details the progress of the Johnson & Johnson Family traveled door-to-door to reach children with ESG Summary 98 of Companies in sustainability. It is also our primary source of VERMOX Chewable tablets for treatment of Performance Data 98 annual disclosure on environmental, social and governance intestinal worms. The medicine is donated GRI Content Index 98 (ESG) performance and should be reviewed in conjunction by Johnson & Johnson and implemented GRI Culture of Health for Business Framework 98 with disclosures on the ESG Policies & Positions page. Data by INMED Partnerships for Children. SASB Index 98 in this Report cover the period between January 1, 2020, and Photo by INMED Partnerships for Children. TCFD 98 December 31, 2020, unless otherwise noted. -

Exhibitor & Sponsor Program
Exhibitor & Sponsor Program www.AtlanticDermConference.org @AtlanticDermADC The Philadelphia Dermatological Society and the Atlantic Derm Conference are pleased to acknowledge and express our sincere appreciation for our corporate sponsors, exhibitors, and commercial supporters. Disclosure of Commercial Support | This activity is supported by an educational donation provided by Amgen, an educational grant provided by Galderma Laboratories, LP, and an educational grant provided by Valeant Dermatology. SPONSORS DIAMOND PLATINUM GOLD SILVER Pharmaceuticals North America LLC www.AtlanticDermConference.org | Page 1 " !"!%! "%#%! !! !#! !% "!! #!!#%$ %"!"! ! !!!! " !%"!! '!!!&! ! "! ' !!! !!"!% "%# ! ( ! # ' % "& ## #!!#" #"$" Exhibitors Exhibitors 3Gen, Inc. | 31521 Rancho Viejo Road, #104, San Juan Capistrano, CA 92675 | 949.481.6384 | www.dermlite.com Table #15 ADDRESSING 3Gen manufactures the DermLite brand of skin imaging devices. THE TOUGHEST AbbVie | 1 N. Waukegan Road, North Chicago, IL 60064 | 847.937.7390 | abbvie.com | www.psoriasis.com Table #1 & 2 AbbVie is a global, research-based biopharmaceutical company which combines the focus of a leading-edge biotech with DERMATOLOGY the expertise and structure of a long-established pharmaceutical leader. AbbVie is committed to using unique approaches to innovation to develop and market advanced therapies that address some of the world’s most complex, serious diseases. CHALLENGES Actelion Pharmaceuticals US, Inc. | 5000 Shoreline Ct., South San Francisco, -

Over-The-Counter (OTC) Medications
Over-the-Counter (OTC) Medications Helpful Tips • Choose products that target your specific symptoms, rather than combination products that treat many symptoms that you may not have. See below. • Be cautious of doubling up on the same ingredients (for example, Tylenol is in many products). • Take extra care when using “PM” products, as they may have serious side effects. • Always read the product labels for important dosing and precaution information. - Doses may differ for adults and children - Many products have age limitations - Most products have a maximum number of days that they should be used OverO-thever--counterthe-counter options options Over-the-Counter Options CombinationCombination Products: Products: treat multipletreat multiple symptoms symptoms Combination products often results in overtreating because you’re taking medications for Combination Products: Treat multiple symptoms Combination• products often results in overtreating because you’re taking medications for • symptoms that you do not have. • Combination products often result in overtreating because you’re taking medications for symptoms that you do not have. Instead,Over choose-the- counteran over-the options-counter p roduct that targets your specific symptoms with symptoms that you do not have. •Instead, choose an over-the-counter product that targets your specific symptoms with • ingredients only for those symptom • Instead, choose an over-the-counter product that targets your specific symptoms with Combination Products:ingredients treat multipleonly for thosesymptoms