Observations on Structure and Evaluation of Possible Functions of the Vexillum in Larval Carapidae (Ophidiiformes)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BONY FISHES 602 Bony Fishes
click for previous page BONY FISHES 602 Bony Fishes GENERAL REMARKS by K.E. Carpenter, Old Dominion University, Virginia, USA ony fishes constitute the bulk, by far, of both the diversity and total landings of marine organisms encoun- Btered in fisheries of the Western Central Atlantic.They are found in all macrofaunal marine and estuarine habitats and exhibit a lavish array of adaptations to these environments. This extreme diversity of form and taxa presents an exceptional challenge for identification. There are 30 orders and 269 families of bony fishes presented in this guide, representing all families known from the area. Each order and family presents a unique suite of taxonomic problems and relevant characters. The purpose of this preliminary section on technical terms and guide to orders and families is to serve as an introduction and initial identification guide to this taxonomic diversity. It should also serve as a general reference for those features most commonly used in identification of bony fishes throughout the remaining volumes. However, I cannot begin to introduce the many facets of fish biology relevant to understanding the diversity of fishes in a few pages. For this, the reader is directed to one of the several general texts on fish biology such as the ones by Bond (1996), Moyle and Cech (1996), and Helfman et al.(1997) listed below. A general introduction to the fisheries of bony fishes in this region is given in the introduction to these volumes. Taxonomic details relevant to a specific family are explained under each of the appropriate family sections. The classification of bony fishes continues to transform as our knowledge of their evolutionary relationships improves. -

Have Chondracanthid Copepods Co-Speciated with Their Teleost Hosts?
Systematic Parasitology 44: 79–85, 1999. 79 © 1999 Kluwer Academic Publishers. Printed in the Netherlands. Have chondracanthid copepods co-speciated with their teleost hosts? Adrian M. Paterson1 & Robert Poulin2 1Ecology and Entomology Group, Lincoln University, PO Box 84, Lincoln, New Zealand 2Department of Zoology, University of Otago, PO Box 56, Dunedin, New Zealand Accepted for publication 26th October, 1998 Abstract Chondracanthid copepods parasitise many teleost species and have a mobile larval stage. It has been suggested that copepod parasites, with free-living infective stages that infect hosts by attaching to their external surfaces, will have co-evolved with their hosts. We examined copepods from the genus Chondracanthus and their teleost hosts for evidence of a close co-evolutionary association by comparing host and parasite phylogenies using TreeMap analysis. In general, significant co-speciation was observed and instances of host switching were rare. The preva- lence of intra-host speciation events was high relative to other such studies and may relate to the large geographical distances over which hosts are spread. Introduction known from the Pacific, and 17 species from the Atlantic (2 species occur in both oceans; none are About one-third of known copepod species are par- reported from the Indian Ocean). asitic on invertebrates or fish (Humes, 1994). The Parasites with direct life-cycles, as well as para- general biology of copepods parasitic on fish is much sites with free-living infective stages that infect hosts better known than that of copepods parasitic on in- by attaching to their external surfaces, are often said to vertebrates (Kabata, 1981). -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

First Record of the Snake Blenny, Ophidion Rochei Müller, 1845 (Actinopterygii: Ophidiiformes: Ophidiidae), from the Sea of Azov
J. Black Sea/Mediterranean Environment Vol. 26, No. 3: 336-342 (2020) SHORT COMMUNICATION First record of the snake blenny, Ophidion rochei Müller, 1845 (Actinopterygii: Ophidiiformes: Ophidiidae), from the Sea of Azov Oleg A. Diripasko*, Viktoriia D. Hetmanenko, Vladislav I. Kyrychenko ORCID IDs: O.A.D. 0000-0001-9267-5504; V.D.H. 0000-0002-2167-7331; V.I.K. 0000-0002-7535-0078 Institute of Fisheries and Marine Ecology, Berdyansk, UKRAINE *Corresponding author: [email protected] Abstract The first record of an Atlantic origin species, the Roche’s snake blenny Ophidion rochei Müller, 1845, in the Black Sea is provided from the south-east Sea of Azov. Its appearance in the Sea of Azov can be explained by the increase in water salinity of the sea in recent years. Keywords: Black Sea, Kerch Strait, first record, distribution, marine fish Received: 17.08.2020, Accepted: 28.11.2020 The Sea of Azov is a small and shallow enclosed sea connected to the north- eastern Black Sea through a narrow strait, the Kerch Strait, and thus belonging to the Mediterranean system of the northern Atlantic Ocean. Its main hydrological feature is low salinity (1–10‰) due to a large freshwater discharge by rivers and limited water exchange with the more saline (17–18‰) Black Sea. Salinity in the Sea of Azov is variable – lower in the large bays adjacent to the Don and Kuban river mouths and higher in the open sea, especially near the Kerch Strait. Besides, it is characterized by long-term fluctuations, both on average and in different areas, due to, mainly, freshwater discharge. -

Evolutionary Affinities of the Unfathomable Parabrotulidae
Molecular Phylogenetics and Evolution 109 (2017) 337–342 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Short Communication Evolutionary affinities of the unfathomable Parabrotulidae: Molecular data indicate placement of Parabrotula within the family Bythitidae, Ophidiiformes ⇑ Matthew A. Campbell a,b, , Jørgen G. Nielsen c, Tetsuya Sado d, Chuya Shinzato e, Miyuki Kanda f, ⇑ Takashi P. Satoh g, Masaki Miya d, a Department of Ecology and Evolutionary Biology, University of California Santa Cruz, Santa Cruz, CA 95064, USA b Fisheries Ecology Division, Southwest Fisheries Science Center, National Marine Fisheries Service, Santa Cruz, CA 95060, USA c Natural History Museum of Denmark, University of Copenhagen, Universitetsparken 15, DK-2100 Copenhagen Ø, Denmark d Department of Ecology and Environmental Sciences, Natural History Museum and Institute, Chiba 260-8682, Japan e Marine Genomics Unit, Okinawa Institute of Science and Technology Graduate University, Okinawa 904-0485, Japan f DNA Sequencing Section, Okinawa Institute of Science and Technology Graduate University, Okinawa 904-0485, Japan g Seto Marine Biological Laboratory, Field Science Education and Research Center, Kyoto University, 459 Shirahama, Nishimuro, Wakayama 649-2211, Japan article info abstract Article history: Fishes are widely diverse in shape and body size and can quite rapidly undergo these changes. Received 3 November 2016 Consequently, some relationships are not clearly resolved with morphological analyses. In the case of Revised 30 January 2017 fishes of small body size, informative characteristics can be absent due to simplification of body struc- Accepted 2 February 2017 tures. The Parabrotulidae, a small family of diminutive body size consisting of two genera and three spe- Available online 6 February 2017 cies has most recently been classified as either a perciform within the suborder Zoarcoidei or an ophidiiform. -

Order Myctophiformes, Lanternfishes
Order Myctophiformes, lanternfishes • 241 species, 35 genera, 2 families • Deep sea pelagic and benthic, numerically dominant in deep sea habitats • Large terminal mouth (reminiscent of anchovy) • Adipose fin present • Compressed head and body (Myctophiformes = nose serpent shape) Lampridiformes • Large eyes Percopsiformes • Photophores Acanthomorpha •Hollow unsegmented spines on dorsal and anal fins •Rostal and premaxilla cartilidge and ligaments allow greater jaw protrusability Order Lampridiformes, opahs and oarfish Order Lampridiformes, opahs and oarfish • Oarfish • 19 species, 12 genera, 7 families – Longest teleost – over 30 feet • no true spines in fins – Only one individual observed • unique upper jaw protrusion – alive, used amiiform maxilla not directly attached to swimming ethmoid or palentine • deep bodied or ribbon-like • pelagic and deep water marine 1 Order Percopsiformes, trout perch, pirate perch, cavefish • 3 families, 7 genera, 9 species • All freshwater • Few with adipose fins – one of the most derived fishes with them • Pirate perch (Aphredoderidae) – One species – Fairly extensive parental care – Anus migration • Cavefish (Amblyopsidae) Zeiformes – Reduction or loss of eyes Gadiformes – Sensory papillae on head, body and tail Acanthomorpha •Hollow unsegmented spines on – Anus migration dorsal and anal fins •Rostal and premaxilla cartilidge – Convergent evolution of cave fish and ligaments allow greater jaw and other cave characins, protrusability catfishes etc. Order Zeiformes Order Gadiiformes • Dories • 555 species, -

SWFSC Archive
Stomiiformes Chapter 4 Order Stomiiformes Number of suborders (2) Gonostomatoidei; Phosichthyoidei (= Photichthyoidei, Stomioidei). Stomiiform monophyly was demonstrated by Fink and Weitzman (1982); relationships within the order are not settled, e.g., Harold and Weitzman (1996). Number of families 4 (or 5: Harold [1998] suggested that Diplophos, Manducus, and Triplophos do not belong in Gonostomatidae, and Nelson [2006] provi sionally placed them in a separate family, Diplophidae). Number of genera 53 Number of species approx. 391 GENERAL LIFE HISTORY REF Distribution All oceans. Relative abundance Rare to very abundant, depending on taxon. Adult habitat Small to medium size (to ca. 10-40 cm) inhabitants of epi-, meso-, and bathypelagic zones, some are vertical migrators. EARLY LIFE HISTORY Mode of reproduction All species known or assumed to be oviparous with planktonic eggs and larvae. Knowledge of ELH Eggs known for 9 genera, larvae known for 38 genera. ELH Characters: Eggs: spherical, ca. 0.6-3.6 mm in diameter, commonly with double membrane, yolk segmented, with 0-1 oil globule ca. 0.1-0.4 mm in di ameter, perivitelline space narrow to wide. Larvae: slender and elongate initially but some become deep-bodied (primarily some sternoptychids and stomiids); preanal length ranges from approx. 30%BL to > 70% BL, most commonly > about half BL, some taxa with trailing gut that can be > 100% BL; eyes strongly ellipti cal to round, most commonly elliptical, stalked in some species; spines lacking on head and pectoral girdle except in some sternoptychids; myo- meres range from 29-164, most commonly approx. mid-30's to mid- 60's; photophores form during postflexion and/or transformation stage; pigmentation absent to heavy, most commonly light. -
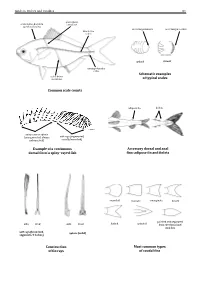
Field Identification Guide to the Living Marine Resources in Kenya
Guide to Orders and Families 81 lateral line scales above scales before dorsal fin outer margin smooth outer margin toothed (predorsal scales) lateral–line 114 scales cycloid ctenoidِّ scales circumpeduncular Schematic examples lateral line of typical scales scales below Common scale counts adipose fin finlets soft rays (segmented, spinyunbranched) rays or spines usually branched) (unsegmented, always Example of a continuous Accessory dorsal and anal dorsal fin of a spiny–rayed fish fins: adipose fin and finlets rounded truncate emarginate lunate side front side front from the dorsal and pointed and separated forked pointed soft rays (branched, spines (solid) segments, 2 halves) anal fins Construction Most common types of fin rays of caudal fins 82 Bony Fishes GUIDE TO ORDERS AND FAMILIES Order ELOPIFORMES – Tarpons and allies Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 23–25 branchiostegal rays; upper jaw extending past eye; tip of snout not overhanging mouth; colour silvery. ELOPIDAE Page 121 very small scales Ladyfishes To 90 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head large mouth gular plate MEGALOPIDAE Page 121 last ray long Tarpons large scales To 55 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head gular plate Order ALBULIFORMES – Bonefishes Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 6–16 branchiostegal rays; upper jaw not extending as far as front of eye; tip of snout overhanging mouth; colour silvery. -

A New Species of the Ophidiid Genus Neobythites (Teleostei: Ophidiiformes) from Tosa Bay, Kochi Prefecture, Japan
Bull. Natl. Mus. Nat. Sci., Ser. A, Suppl. 6, pp. 27–32, March 30, 2012 A New Species of the Ophidiid Genus Neobythites (Teleostei: Ophidiiformes) from Tosa Bay, Kochi Prefecture, Japan Shinpei Ohashi1, Jørgen G. Nielsen2 and Mamoru Yabe3 1 Chair of Marine Biology and Biodiversity (Systematic Ichthyology), Graduate School of Fisheries Sciences, Hokkaido University, 3–1–1 Minato-cho, Hakodate, Hokkaido 041–8611, Japan E-mail: shin-ohashi@¿sh.hokudai.ac.jp 2 Natural History Museum of Denmark, Universitetsparken 15, DK-2100 Copenhagen, Denmark E-mail: [email protected] 3 Laboratory of Marine Biology and Biodiversity (Systematic Ichthyology), Research Faculty of Fisheries Sciences, Hokkaido University, Hakodate, Hokkaido 041–8611, Japan E-mail: myabe@¿sh.hokudai.ac.jp Abstract A new ophidiid species, Neobythites machidai, is based on 7 specimens (63.0–93.5 mm SL), collected from Tosa Bay (139–176 m depth), Kochi Prefecture, southern Japan. It is most sim- ilar to N. bimarginatus, known from off New Caledonia, by having many pectoral-¿n rays (>30), preopercle without spines and black bands in middle part of dorsal and anal ¿ns. However, N. machidai differs from N. bimarginatus by pelvic-¿n length 8.5–11.5% SL (vs. 11.5–13.5% SL in the latter species), longest gill ¿lament 6.9–10.0% HL (vs. 4.8–6.3% HL), each side of triangular vomerine tooth patch concave (vs. slightly convex), snout shorter than horizontal eye window (vs. snout longer than eye), and 11–13 (vs. 6–7) light spots on middle part of body. Additionally, they differ in many characters such as number of dorsal-¿n rays, pectoral-¿n rays and total vertebrae and preanal length. -

The Exceptional Diversity of Visual Adaptations in Deep-Sea Teleost Fishes
Seminars in Cell and Developmental Biology xxx (xxxx) xxx–xxx Contents lists available at ScienceDirect Seminars in Cell & Developmental Biology journal homepage: www.elsevier.com/locate/semcdb Review The exceptional diversity of visual adaptations in deep-sea teleost fishes Fanny de Busserolles*, Lily Fogg, Fabio Cortesi, Justin Marshall Queensland Brain Institute, The University of Queensland, St Lucia, Queensland 4072, Australia ARTICLE INFO ABSTRACT Keywords: The deep-sea is the largest and one of the dimmest habitats on earth. In this extreme environment, every photon Deep-sea teleost counts and may make the difference between life and death for its inhabitants. Two sources of light are present Dim-light vision in the deep-sea; downwelling light, that becomes dimmer and spectrally narrower with increasing depth until Ocular adaptation completely disappearing at around 1000 m, and bioluminescence, the light emitted by animals themselves. Retina Despite these relatively dark and inhospitable conditions, many teleost fish have made the deep-sea their home, Opsin relying heavily on vision to survive. Their visual systems have had to adapt, sometimes in astonishing and Bioluminescence bizarre ways. This review examines some aspects of the visual system of deep-sea teleosts and highlights the exceptional diversity in both optical and retinal specialisations. We also reveal how widespread several of these adaptations are across the deep-sea teleost phylogeny. Finally, the significance of some recent findings as well as the surprising diversity in visual adaptations is discussed. 1. Introduction or mate detection, to communicate, camouflage, or for navigation, in- cluding to stay within a particular depth range [4,5]. -

Guide to Some Trawl-Caught Marine Fishes from Maine to Cape Hatteras, North Carolina
431 NOAA Technical Report NMFS Circular 431 Guide to Some Trawl-Caught Marine Fishes From Maine to Cape Hatteras, North Carolina Donald D. Flescher March 1980 U.S . DEPARTMENT OF COMMERCE National Oceanic and Atmospheric Administration National Marine Fisheries Service NOAA TECHNICAL REPORTS National Marine Fisheries Service, Circulars The major responsibiilties III the "attllnol \Iann.' FI,hpnp, ",'rvllP I:-':\HSI ArI' t(, m(,ml"r snd delhI aIJundan(t and j(Pfll(raphlf dlStrihution IIf lisherv r~sources, til unlierslunli snd prelil' I flUCI'.I1t1 ",ns In t hi' 4U n',1\ and d, trJbut H'n (,I Ihe < re rune and til E' labli h leHI lor optImum use of the resour('es, ,\:\IF:-; IS also (harged wllh Ih., d,'v!'lopmenl and ,mT,lementst,,·n "I poil" , I'lf man gllll( nallonal II hlnl( grounds, development and pnfoflempnt 01 dome,t'l Ii,hen," rpgu~AtH·ns, "If\(,1;18nCI' (of f'·rell(n f, h nj( "ft I nl'('(l"l I" ('Ia till "dler ann thE' development and enlorcement "I IllternatillnallJsh.n ul(rf't'menls and pollCl"s .... :\1~" .,1,,· a" I Ihe" hlng Ind •• Ir thr"ul'h rnarhltnj( ef\ ce and e(,lInllmlt anal\", prClgnl'n,. and ""rtgage n, Han' e and \(,,,(,1 (·m trurtll" L,h"d'e It, (" e I anal\le lnd puh he \3f1I1US phases "f the Indust r\ Tht' ,\OAA Technical Hpp"rt '\IF:-. t'Jr(ular "'rJ!" r m',n Ie'" ,eflt th,,' hft heN, n ( le'He ,net'. II. The (,r- 11M publicallons "I general'l1lt'resl Inlennt'ri I" ~J(! (lI"pr\.lIH·O and manag('rr(nl Puhl" tl' In 'Ilat rt"~" .n ('Ill lripnhp dr'~" lechnl(alleHI cerlaln hn an arfd' It research ~T:)ear in 'hi ,tnt· I fllln 01 paper. -

A New Pearlfish, Onuxodon Albometeori Sp. Nov. (Ophidiiformes: Carapidae), from Taiwan
Zootaxa 4702 (1): 006–009 ISSN 1175-5326 (print edition) https://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2019 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4702.1.4 http://zoobank.org/urn:lsid:zoobank.org:pub:515DA6FB-A724-4D77-94F9-62EB513AB454 A new pearlfish, Onuxodon albometeori sp. nov. (Ophidiiformes: Carapidae), from Taiwan KEITA KOEDA1,2 1National Museum of Marine Biology & Aquarium, 2 Houwan Road, Checheng, Pingtung 94450, Taiwan 2Present address: Kuroshio Biological Research Foundation, 560 Nishidomari, Otsuki, Kochi 788-0333, Japan Corresponding author. E-mail: [email protected] Abstract Onuxodon albometeori sp. nov. (Ophidiiformes: Carapidae) is described from a single specimen collected by commercial trawl off southwestern Taiwan. The new species is most similar to the Indo-Pacific species Onuxodon fowleri (Smith 1955), both process a remarkably slender body, and higher precaudal vertebral counts and a longer pectoral fin, although the two latter features are even more extreme in the former. Onuxodon albometeori sp. nov. is further distinguished from O. fowleri by its lesser body depth, greater head width, higher counts of precaudal vertebrae, and uniformly whitish coloration only on the posterior part of the body. Key words: Onuxodon fowleri, taxonomy, morphology, new species, symbiotic organism Introduction The pearlfish family Carapidae is characterized by small translucent slender bodies and can easily escape notice due to the cryptic life style. The adults of most species live symbiotically or hide inside various invertebrates. The genus Onuxodon Smith 1955 comprises four nominal species, three of them are recognized as valid: Onuxodon fowleri (Smith 1955), Onuxodon margaritiferae (Rendahl 1921) and Onuxodon parvibrachium (Fowler 1927); all distributed in the Indo-Pacific region (Markle and Olney 1990).