On the Origin and Morphology of Myoepithelial Cells of Apocrine Sweat Glands* D J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Expression of Integrins and Basement Membrane Components by Wound Keratinocytes
Expression of integrins and basement membrane components by wound keratinocytes. H Larjava, … , R H Kramer, J Heino J Clin Invest. 1993;92(3):1425-1435. https://doi.org/10.1172/JCI116719. Research Article Extracellular matrix proteins and their cellular receptors, integrins, play a fundamental role in keratinocyte adhesion and migration. During wound healing, keratinocytes detach, migrate until the two epithelial sheets confront, and then regenerate the basement membrane. We examined the expression of different integrins and their putative ligands in keratinocytes during human mucosal wound healing. Migrating keratinocytes continuously expressed kalinin but not the other typical components of the basement membrane zone: type IV collagen, laminin, and type VII collagen. When the epithelial sheets confronted each other, these missing basement membrane components started to appear gradually through the entire wound area. The expression of integrin beta 1 subunit was increased in keratinocytes during migration. The beta 1-associated alpha 2 and alpha 3 subunits were expressed constantly by wound keratinocytes whereas the alpha 5 subunit was present only in keratinocytes during reepithelialization. Furthermore, migrating cells started to express alpha v-integrins which were not present in the nonaffected epithelium. All keratinocytes also expressed the alpha 6 beta 4 integrin during migration. In the migrating cells, the distribution of integrins was altered. In normal mucosa, beta 1-integrins were located mainly on the lateral plasma membrane and alpha 6 beta 4 at the basal surface of basal keratinocytes in the nonaffected tissue. In wounds, integrins were found in filopodia of migrating keratinocytes, and also surrounding cells in […] Find the latest version: https://jci.me/116719/pdf Expression of Integrins and Basement Membrane Components by Wound Keratinocytes Hannu Lariava, * Tuula Salo,t Kirsi Haapasalmi, * Randall H. -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Modelling Breast Epithelial-Endothelial Interaction in Three-Dimensional Cell Culture
Modelling breast epithelial-endothelial interaction in three-dimensional cell culture A thesis submitted for the degree of Master of Science Sævar Ingþórsson Department of Medicine University of Iceland Instructors and Masters Project Committee: Þórarinn Guðjónsson, Ph.D Magnús Karl Magnússon, MD Kristján Leósson, Ph.D Reykjavik, Iceland September 2008 Samspil æðaþels og eðlilegs og illkynja þekjuvefjar úr brjóstkirtli í þrívíðri frumurækt Ritgerð til meistaragráðu Sævar Ingþórsson Háskóli Íslands Læknadeild Leiðbeinendur og meistaranámsnefnd: Þórarinn Guðjónsson, Ph.D Magnús Karl Magnússon, MD Kristján Leósson, Ph.D Reykjavík, September 2008 Ágrip Brjóstkirtillinn samanstendur af tveimur megingerðum þekjuvefsfruma, kirtilþekju- og vöðvaþekjufrumum. Saman mynda þessar frumugerðir hina greinóttu formgerð brjóstkirtilsins. Kirtilvefurinn er umlukinn æðaríkum stoðvef sem inniheldur margar mismunandi frumugerðir, þ.m.t. bandvefsfrumur og æðaþelsfrumur. Þroskun og sérhæfing kirtilsins er mjög háð samskiptum hans við millifrumuefni brjóstsins og frumur stoðvefjarins. Mest áhersla hefur verið lögð á rannsóknir á bandvefsfrumum í þessu tilliti, en minni athygli beint að æðaþelsfrumum, sem voru lengi taldar gegna því hlutverki einu að miðla súrefni og næringu um líkamann. Á síðustu árum hefur verið sýnt fram á að nýmyndun æða í krabbameinsæxlum spili stórt hlutverk í framþróun æxlisvaxtar og hefur það verið tengt slæmum horfum. Nýlegar rannsóknir hafa sýnt fram á mikilvægt hlutverk æðaþels í þroskun og sérhæfingu ýmissa líffæra, til dæmis í heila, lifur og beinmerg sem og í framþróun krabbameins. Nýleg þekking bendir einnig til mikilvægra áhrifa æðaþels á þroskun eðlilegs og illkynja brjóstvefjar. Markmið verkefnisins er að kanna áhrif brjóstaæðaþels á eðlilegar og illkynja brjóstaþekjufrumulínur og nota til þess þrívíð ræktunarlíkön sem þróuð voru á rannsóknastofunni, sem og að endurbæta þessi líkön til frekari rannsókna á samskiptum æðaþels og þekjufruma. -

An Analysis of Benign Human Prostate Offers Insights Into the Mechanism
www.nature.com/scientificreports OPEN An analysis of benign human prostate ofers insights into the mechanism of apocrine secretion Received: 12 March 2018 Accepted: 22 February 2019 and the origin of prostasomes Published: xx xx xxxx Nigel J. Fullwood 1, Alan J. Lawlor2, Pierre L. Martin-Hirsch3, Shyam S. Matanhelia3 & Francis L. Martin 4 The structure and function of normal human prostate is still not fully understood. Herein, we concentrate on the diferent cell types present in normal prostate, describing some previously unreported types and provide evidence that prostasomes are primarily produced by apocrine secretion. Patients (n = 10) undergoing TURP were prospectively consented based on their having a low risk of harbouring CaP. Scanning electron microscopy and transmission electron microscopy was used to characterise cell types and modes of secretion. Zinc levels were determined using Inductively Coupled Plasma Mass Spectrometry. Although merocrine secretory cells were noted, the majority of secretory cells appear to be apocrine; for the frst time, we clearly show high-resolution images of the stages of aposome secretion in human prostate. We also report a previously undescribed type of epithelial cell and the frst ultrastructural image of wrapping cells in human prostate stroma. The zinc levels in the tissues examined were uniformly high and X-ray microanalysis detected zinc in merocrine cells but not in prostasomes. We conclude that a signifcant proportion of prostasomes, possibly the majority, are generated via apocrine secretion. This fnding provides an explanation as to why so many large proteins, without a signal peptide sequence, are present in the prostatic fuid. Tere are many complications associated with the prostate from middle age onwards, including benign prostatic hyperplasia (BPH) and prostate cancer (PCa). -

Sweat Gland Myoepithelial Cell Differentiation
Journal of Cell Science 112, 1925-1936 (1999) 1925 Printed in Great Britain © The Company of Biologists Limited 1999 JCS4638 Human sweat gland myoepithelial cells express a unique set of cytokeratins and reveal the potential for alternative epithelial and mesenchymal differentiation states in culture Margarete Schön1,*, Jennifer Benwood1, Therese O’Connell-Willstaedt2 and James G. Rheinwald1,2,‡ 1Division of Dermatology/Department of Medicine, Brigham and Women’s Hospital, and 2Division of Cell Growth and Regulation, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA 02115, USA *Present address: Department of Dermatology, Heinrich-Heine University, Moorenstrasse 5, 40225 Düsseldorf, Germany ‡Author for correspondence (e-mail: [email protected]) Accepted 9 April; published on WWW 26 May 1999 SUMMARY We have characterized precisely the cytokeratin expression myoepithelial cells, a constituent of secretory glands. pattern of sweat gland myoepithelial cells and have Immunostaining of skin sections revealed that only sweat identified conditions for propagating this cell type and gland myoepithelial cells expressed the same pattern of modulating its differentiation in culture. Rare, unstratified keratins and α-sma and lack of E-cadherin as the cell type epithelioid colonies were identified in cultures initiated we had cultured. Interestingly, our immunocytochemical from several specimens of full-thickness human skin. These analysis of ndk, a skin-derived cell line of uncertain cells divided rapidly in medium containing serum, identity, suggests that this line is of myoepithelial origin. epidermal growth factor (EGF), and hydrocortisone, and Earlier immunohistochemical studies by others had found maintained a closely packed, epithelioid morphology when myoepithelial cells to be K7-negative. -
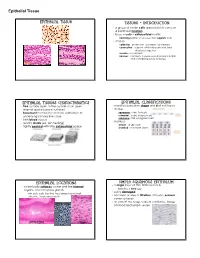
Epithelial Tissue
Epithelial Tissue Epithelial Tissue Tissues - Introduction · a group of similar cells specialized to carry on a particular function · tissue = cells + extracellular matrix nonliving portion of a tissue that supports cells · 4 types epithelial - protection, secretion, absorption connective - support soft body parts and bind structures together muscle - movement nervous - conducts impulses used to help control and coordinate body activities Epithelial Tissues Characteristics Epithelial Classifications · free surface open to the outside or an open · classified based on shape and # of cell layers internal space (apical surface) · shape · basement membrane anchors epithelium to squamous - thin, flat cells underlying connective tissue cuboidal - cube-shaped cells columnar - tall, elongated cells · lack blood vessels · number · readily divide (ex. skin healing) simple - single layer · tightly packed with little extracellular space stratified - 2 or more layers Epithelial Locations Simple Squamous Epithelium · a single layer of thin, flattened cells · cover body surfaces, cover and line internal organs, and compose glands looks like a fried egg · easily damaged skin cells, cells that line the stomach and small intestine, inside your mouth · common at sites of filtration, diffusion, osmosis; cover surfaces · air sacs of the lungs, walls of capillaries, linings cheek cells of blood and lymph vessels intestines skin Epithelial Tissue Simple Cuboidal Epithelium Simple Columnar Epithelium · single layer of cube-shaped cells · single layer of cells -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Nails Develop from Thickened Areas of Epidermis at the Tips of Each Digit Called Nail Fields
Nail Biology: The Nail Apparatus Nail plate Proximal nail fold Nail matrix Nail bed Hyponychium Nail Biology: The Nail Apparatus Lies immediately above the periosteum of the distal phalanx The shape of the distal phalanx determines the shape and transverse curvature of the nail The intimate anatomic relationship between nail and bone accounts for the bone alterations in nail disorders and vice versa Nail Apparatus: Embryology Nail field develops during week 9 from the epidermis of the dorsal tip of the digit Proximal border of the nail field extends downward and proximally into the dermis to create the nail matrix primordium By week 15, the nail matrix is fully developed and starts to produce the nail plate Nails develop from thickened areas of epidermis at the tips of each digit called nail fields. Later these nail fields migrate onto the dorsal surface surrounded laterally and proximally by folds of epidermis called nail folds. Nail Func7on Protect the distal phalanx Enhance tactile discrimination Enhance ability to grasp small objects Scratching and grooming Natural weapon Aesthetic enhancement Pedal biomechanics The Nail Plate Fully keratinized structure produced throughout life Results from maturation and keratinization of the nail matrix epithelium Attachments: Lateral: lateral nail folds Proximal: proximal nail fold (covers 1/3 of the plate) Inferior: nail bed Distal: separates from underlying tissue at the hyponychium The Nail Plate Rectangular and curved in 2 axes Transverse and horizontal Smooth, although -

Anatomy of the Gallbladder and Bile Ducts
BASIC SCIENCE the portal vein lies posterior to these structures; Anatomy of the gallbladder the inferior vena cava, separated by the epiploic foramen (the foramen of Winslow) lies still more posteriorly, and bile ducts behind the portal vein. Note that haemorrhage during gallbladder surgery may be Harold Ellis controlled by compression of the hepatic artery, which gives off the cystic branch, by passing a finger through the epiploic foramen (foramen of Winslow), and compressing the artery Abstract between the finger and the thumb placed on the anterior aspect A detailed knowledge of the gallbladder and bile ducts (together with of the foramen (Pringle’s manoeuvre). their anatomical variations) and related blood supply are essential in At fibreoptic endoscopy, the opening of the duct of Wirsung the safe performance of both open and laparoscopic cholecystectomy can usually be identified quite easily. It is seen as a distinct as well as the interpretation of radiological and ultrasound images of papilla rather low down in the second part of the duodenum, these structures. These topics are described and illustrated. lying under a characteristic crescentic mucosal fold (Figure 2). Unless the duct is obstructed or occluded, bile can be seen to Keywords Anatomical variations; bile ducts; blood supply; gallbladder discharge from it intermittently. The gallbladder (Figures 1 and 3) The biliary ducts (Figure 1) The normal gallbladder has a capacity of about 50 ml of bile. It concentrates the hepatic bile by a factor of about 10 and also The right and left hepatic ducts emerge from their respective sides secretes mucus into it from the copious goblet cells scattered of the liver and fuse at the porta hepatis (‘the doorway to the throughout its mucosa. -

Histological Variations in Myoepithelial Cells and Arrectores Pilorum Muscles Among Caudal, Metatarsal and Preorbital Glands In
NOTE Anatomy Histological Variations in Myoepithelial Cells and Arrectores Pilorum Muscles among Caudal, Metatarsal and Preorbital Glands in Hokkaido Sika Deer (Cervus nippon yesoensis Heude, 1884) Nobuo OZAKI1), Masatsugu SUZUKI1)* and Noriyuki OHTAISHI1) 1)Laboratory of Wildlife Biology, The Graduate School of Veterinary Medicine, Hokkaido University, Kita-ku, Sapporo 060–0818, Japan (Received 1 April 2003/Accepted 15 October 2003) ABSTRACT. The morphological characteristics of myoepithelial cells and arrectores pilorum muscles were investigated in caudal, metatarsal and preorbital glands of Hokkaido sika deer (Cervus nippon yesoensis Heude, 1884) using immunohistochemistry for α-smooth muscle actin. In the metatarsal, preorbital and general skin glands, myoepithelial cell layers continuously embraced the secretory epithelium, while in the caudal gland, discontinuous myoepithelial cell rows surrounded the apocrine tubules. There was a trend that the widths of the myoepithelial cells of the caudal and preorbital glands appeared to be thinner than those of the metatarsal and general skin glands. In the metatarsal gland, the arrectores pilorum muscles were highly developed and considerably larger than those in other skin glands. KEY WORDS: arrectores pilorum muscle, myoepithelial cell, sika deer. J. Vet. Med. Sci. 66(3): 283–285, 2004 The specialized skin glands in the cervid species include were incubated for 15 min at room temperature with a bioti- forehead, preorbital, tarsal, metatarsal, interdigital and cau- nylated rabbit antibody against mouse IgG, IgA and IgM dal glands [15], most of which contain both apocrine and (Nichirei). Then they were rinsed three times (10 min each) sebaceous glandular elements [1, 7, 12]. In Hokkaido sika in PBS and incubated for 10 min at room temperature in a deer (Cervus nippon yesoensis Heude, 1884), the existence streptavidin-biotin peroxidase complex (Nichirei). -

Basement Membrane
J Clin Pathol: first published as 10.1136/jcp.s3-12.1.59 on 1 January 1978. Downloaded from J. clin. Path., 31, Suppl. (Roy. Coll. Path.), 12, 59-66 Basement membrane JOHN T. IRELAND From the Southern General Hospital, Glasgow, and the University of Glasgow Basement membrane has attracted less attention will be reviewed with the aim of providing new than the other components of connective tissue. But, insight into the function of basement membrane in like the other extracellular fibres and matrix health and disease. materials, it can profoundly influence both the structure and function of advanced life forms. Basement membrane turnover Usually taking the shape of thin, structureless cementing material between epithelial and con- Much evidence has accumulated from various nective tissue, basement membranes are widely sources (Farquhar et al., 1961; Andres et al., 1962; distributed throughout the body. They are found Kurtz and Feldman, 1962; Vernier, 1964; Pierce, as extracellular components of blood capillaries, 1966; Thoenes, 1967; Lee et al., 1969; Walker, 1973) renal glomeruli and tubules, alveoli, retina, lens to show that visceral epithelium is responsible for capsule, muscle parasarcolemma, sweat glands, basement membrane synthesis (Fig. 2). Until recently, Schwann cells, and breast ducts. Although sharing however, its rate of turnover had been less clear. The similar morphological, biochemical, and antigenic elegant, long-termsequentialstudiesof Walker (1973), properties, basement membrane can be developed using the argyric technique, confirmed the view that for different functions in special situations. In the turnover is slow. Clearance of silver from the rat lens capsule of the eye, for example, it provides glomerulus is exclusively undirectional from the copyright.