A Mesozoic Bird from Gondwana Preserving Feathers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Phylogenetic Position of Ambiortus: Comparison with Other Mesozoic Birds from Asia1 J
ISSN 00310301, Paleontological Journal, 2013, Vol. 47, No. 11, pp. 1270–1281. © Pleiades Publishing, Ltd., 2013. The Phylogenetic Position of Ambiortus: Comparison with Other Mesozoic Birds from Asia1 J. K. O’Connora and N. V. Zelenkovb aKey Laboratory of Evolution and Systematics, Institute of Vertebrate Paleontology and Paleoanthropology, 142 Xizhimenwai Dajie, Beijing China 10044 bBorissiak Paleontological Institute, Russian Academy of Sciences, Profsoyuznaya ul. 123, Moscow, 117997 Russia email: [email protected], [email protected] Received August 6, 2012 Abstract—Since the last description of the ornithurine bird Ambiortus dementjevi from Mongolia, a wealth of Early Cretaceous birds have been discovered in China. Here we provide a detailed comparison of the anatomy of Ambiortus relative to other known Early Cretaceous ornithuromorphs from the Chinese Jehol Group and Xiagou Formation. We include new information on Ambiortus from a previously undescribed slab preserving part of the sternum. Ambiortus is superficially similar to Gansus yumenensis from the Aptian Xiagou Forma tion but shares more morphological features with Yixianornis grabaui (Ornithuromorpha: Songlingorni thidae) from the Jiufotang Formation of the Jehol Group. In general, the mosaic pattern of character distri bution among early ornithuromorph taxa does not reveal obvious relationships between taxa. Ambiortus was placed in a large phylogenetic analysis of Mesozoic birds, which confirms morphological observations and places Ambiortus in a polytomy with Yixianornis and Gansus. Keywords: Ornithuromorpha, Ambiortus, osteology, phylogeny, Early Cretaceous, Mongolia DOI: 10.1134/S0031030113110063 1 INTRODUCTION and articulated partial skeleton, preserving several cervi cal and thoracic vertebrae, and parts of the left thoracic Ambiortus dementjevi Kurochkin, 1982 was one of girdle and wing (specimen PIN, nos. -

Onetouch 4.0 Scanned Documents
/ Chapter 2 THE FOSSIL RECORD OF BIRDS Storrs L. Olson Department of Vertebrate Zoology National Museum of Natural History Smithsonian Institution Washington, DC. I. Introduction 80 II. Archaeopteryx 85 III. Early Cretaceous Birds 87 IV. Hesperornithiformes 89 V. Ichthyornithiformes 91 VI. Other Mesozojc Birds 92 VII. Paleognathous Birds 96 A. The Problem of the Origins of Paleognathous Birds 96 B. The Fossil Record of Paleognathous Birds 104 VIII. The "Basal" Land Bird Assemblage 107 A. Opisthocomidae 109 B. Musophagidae 109 C. Cuculidae HO D. Falconidae HI E. Sagittariidae 112 F. Accipitridae 112 G. Pandionidae 114 H. Galliformes 114 1. Family Incertae Sedis Turnicidae 119 J. Columbiformes 119 K. Psittaciforines 120 L. Family Incertae Sedis Zygodactylidae 121 IX. The "Higher" Land Bird Assemblage 122 A. Coliiformes 124 B. Coraciiformes (Including Trogonidae and Galbulae) 124 C. Strigiformes 129 D. Caprimulgiformes 132 E. Apodiformes 134 F. Family Incertae Sedis Trochilidae 135 G. Order Incertae Sedis Bucerotiformes (Including Upupae) 136 H. Piciformes 138 I. Passeriformes 139 X. The Water Bird Assemblage 141 A. Gruiformes 142 B. Family Incertae Sedis Ardeidae 165 79 Avian Biology, Vol. Vlll ISBN 0-12-249408-3 80 STORES L. OLSON C. Family Incertae Sedis Podicipedidae 168 D. Charadriiformes 169 E. Anseriformes 186 F. Ciconiiformes 188 G. Pelecaniformes 192 H. Procellariiformes 208 I. Gaviiformes 212 J. Sphenisciformes 217 XI. Conclusion 217 References 218 I. Introduction Avian paleontology has long been a poor stepsister to its mammalian counterpart, a fact that may be attributed in some measure to an insufRcien- cy of qualified workers and to the absence in birds of heterodont teeth, on which the greater proportion of the fossil record of mammals is founded. -

New Oviraptorid Dinosaur (Dinosauria: Oviraptorosauria) from the Nemegt Formation of Southwestern Mongolia
Bull. Natn. Sci. Mus., Tokyo, Ser. C, 30, pp. 95–130, December 22, 2004 New Oviraptorid Dinosaur (Dinosauria: Oviraptorosauria) from the Nemegt Formation of Southwestern Mongolia Junchang Lü1, Yukimitsu Tomida2, Yoichi Azuma3, Zhiming Dong4 and Yuong-Nam Lee5 1 Institute of Geology, Chinese Academy of Geological Sciences, Beijing 100037, China 2 National Science Museum, 3–23–1 Hyakunincho, Shinjukuku, Tokyo 169–0073, Japan 3 Fukui Prefectural Dinosaur Museum, 51–11 Terao, Muroko, Katsuyama 911–8601, Japan 4 Institute of Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing 100044, China 5 Korea Institute of Geoscience and Mineral Resources, Geology & Geoinformation Division, 30 Gajeong-dong, Yuseong-gu, Daejeon 305–350, South Korea Abstract Nemegtia barsboldi gen. et sp. nov. here described is a new oviraptorid dinosaur from the Late Cretaceous (mid-Maastrichtian) Nemegt Formation of southwestern Mongolia. It differs from other oviraptorids in the skull having a well-developed crest, the anterior margin of which is nearly vertical, and the dorsal margin of the skull and the anterior margin of the crest form nearly 90°; the nasal process of the premaxilla being less exposed on the dorsal surface of the skull than those in other known oviraptorids; the length of the frontal being approximately one fourth that of the parietal along the midline of the skull. Phylogenetic analysis shows that Nemegtia barsboldi is more closely related to Citipati osmolskae than to any other oviraptorosaurs. Key words : Nemegt Basin, Mongolia, Nemegt Formation, Late Cretaceous, Oviraptorosauria, Nemegtia. dae, and Caudipterygidae (Barsbold, 1976; Stern- Introduction berg, 1940; Currie, 2000; Clark et al., 2001; Ji et Oviraptorosaurs are generally regarded as non- al., 1998; Zhou and Wang, 2000; Zhou et al., avian theropod dinosaurs (Osborn, 1924; Bars- 2000). -

Magnificent Magpie Colours by Feathers with Layers of Hollow Melanosomes Doekele G
© 2018. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2018) 221, jeb174656. doi:10.1242/jeb.174656 RESEARCH ARTICLE Magnificent magpie colours by feathers with layers of hollow melanosomes Doekele G. Stavenga1,*, Hein L. Leertouwer1 and Bodo D. Wilts2 ABSTRACT absorption coefficient throughout the visible wavelength range, The blue secondary and purple-to-green tail feathers of magpies are resulting in a higher refractive index (RI) than that of the structurally coloured owing to stacks of hollow, air-containing surrounding keratin. By arranging melanosomes in the feather melanosomes embedded in the keratin matrix of the barbules. barbules in more or less regular patterns with nanosized dimensions, We investigated the spectral and spatial reflection characteristics of vivid iridescent colours are created due to constructive interference the feathers by applying (micro)spectrophotometry and imaging in a restricted wavelength range (Durrer, 1977; Prum, 2006). scatterometry. To interpret the spectral data, we performed optical The melanosomes come in many different shapes and forms, and modelling, applying the finite-difference time domain (FDTD) method their spatial arrangement is similarly diverse (Prum, 2006). This has as well as an effective media approach, treating the melanosome been shown in impressive detail by Durrer (1977), who performed stacks as multi-layers with effective refractive indices dependent on extensive transmission electron microscopy of the feather barbules the component media. The differently coloured magpie feathers are of numerous bird species. He interpreted the observed structural realised by adjusting the melanosome size, with the diameter of the colours to be created by regularly ordered melanosome stacks acting melanosomes as well as their hollowness being the most sensitive as optical multi-layers. -

The Molecular Evolution of Feathers with Direct Evidence from Fossils
The molecular evolution of feathers with direct evidence from fossils Yanhong Pana,1, Wenxia Zhengb, Roger H. Sawyerc, Michael W. Penningtond, Xiaoting Zhenge,f, Xiaoli Wange,f, Min Wangg,h, Liang Hua,i, Jingmai O’Connorg,h, Tao Zhaoa, Zhiheng Lig,h, Elena R. Schroeterb, Feixiang Wug,h, Xing Xug,h, Zhonghe Zhoug,h,i,1, and Mary H. Schweitzerb,j,1 aChinese Academy of Sciences Key Laboratory of Economic Stratigraphy and Palaeogeography, Nanjing Institute of Geology and Palaeontology and Center for Excellence in Life and Paleoenvironment, Chinese Academy of Sciences, Nanjing 210008, China; bDepartment of Biological Sciences, North Carolina State University, Raleigh, NC 27695; cDepartment of Biological Sciences, University of South Carolina, Columbia, SC 29205; dAmbioPharm Incorporated, North Augusta, SC 29842; eInstitute of Geology and Paleontology, Lingyi University, Lingyi City, 27605 Shandong, China; fShandong Tianyu Museum of Nature, Pingyi, 273300 Shandong, China; gCAS Key Laboratory of Vertebrate Evolution and Human Origins of the Chinese Academy of Sciences, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, 100044 Beijing, China; hCenter for Excellence in Life and Paleoenvironment, Chinese Academy of Sciences, 100044 Beijing, China; iCollege of Earth and Planetary Sciences, University of Chinese Academy of Sciences, 100049 Beijing, China; and jNorth Carolina Museum of Natural Sciences, Raleigh, NC 27601 Contributed by Zhonghe Zhou, December 15, 2018 (sent for review September 12, 2018; reviewed by Dominique G. Homberger and Chenxi Jia) Dinosaur fossils possessing integumentary appendages of various feathers in Anchiornis, barbules that interlock to form feather morphologies, interpreted as feathers, have greatly enhanced our vanes critical for flight have not been identified yet (12). -
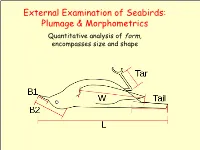
External Examination of Seabirds: Plumage & Morphometrics
External Examination of Seabirds: Plumage & Morphometrics Quantitative analysis of form, encompasses size and shape Seabird Topography ➢ Naming Conventions: • Parts of the body • Types of feathers (Harrison 1983) Types of Feathers Coverts: Rows bordering and overlaying the edges of the tail and wings on both the lower and upper sides of the body. Help streamline shape of the wings and tail and provide the bird with insulation. Feather Tracks ➢ Feathers are not attached randomly. • They occur in linear tracts called pterylae. • Spaces on bird's body without feather tracts are called apteria. • Densest area for feather tracks is head and neck. • Feathers arranged in distinct layers: contour feathers overlay down. Generic Pterylae Types of Feathers Contour feathers: outermost feathers. Define the color and shape of the bird. Contour feathers lie on top of each other, like shingles on a roof. Shed water, keeping body dry and insulated. Each contour feather controlled by specialized muscles which control their position, allowing the bird to keep the feathers in clean and neat condition. Specialized contour feathers used for flight: delineate outline of wings and tail. Types of Feathers Flight feathers – special contour feathers Define outline of wings and tail Long and stiff Asymmetrical those on wings are called remiges (singular remex) those on tail are called retrices (singular retrix) Types of Flight Feathers Remiges: Largest contour feathers (primaries / secondaries) Responsible for supporting bird during flight. Attached by ligaments or directly to the wing bone. Types of Flight Feathers Flight feathers – special contour feathers Rectrices: tail feathers provide flight stability and control. Connected to each other by ligaments, with only the inner- most feathers attached to bone. -

Reidentification of Avian Embryonic Remains from the Cretaceous of Mongolia
RESEARCH ARTICLE Reidentification of Avian Embryonic Remains from the Cretaceous of Mongolia David J. Varricchio1*, Amy M. Balanoff2, Mark A. Norell3 1 Department of Earth Sciences, Montana State University, Bozeman, Montana, 59717, United States of America, 2 Department of Anatomical Sciences, Stony Brook University School of Medicine, Stony Brook, NY, 11794, United States of America, 3 Division of Paleontology, American Museum of Natural History, New York, NY, 10024, United States of America * [email protected] Abstract Embryonic remains within a small (4.75 by 2.23 cm) egg from the Late Cretaceous, Mongo- lia are here re-described. High-resolution X-ray computed tomography (HRCT) was used to digitally prepare and describe the enclosed embryonic bones. The egg, IGM (Mongolian In- stitute for Geology, Ulaanbaatar) 100/2010, with a three-part shell microstructure, was originally assigned to Neoceratopsia implying extensive homoplasy among eggshell char- acters across Dinosauria. Re-examination finds the forelimb significantly longer than the hindlimbs, proportions suggesting an avian identification. Additional, postcranial apomor- phies (strut-like coracoid, cranially located humeral condyles, olecranon fossa, slender radi- OPEN ACCESS us relative to the ulna, trochanteric crest on the femur, and ulna longer than the humerus) identify the embryo as avian. Presence of a dorsal coracoid fossa and a craniocaudally Citation: Varricchio DJ, Balanoff AM, Norell MA (2015) Reidentification of Avian Embryonic Remains compressed distal humerus with a strongly angled distal margin support a diagnosis of IGM from the Cretaceous of Mongolia. PLoS ONE 10(6): 100/2010 as an enantiornithine. Re-identification eliminates the implied homoplasy of this e0128458. doi:10.1371/journal.pone.0128458 tri-laminate eggshell structure, and instead associates enantiornithine birds with eggshell Academic Editor: Peter Dodson, University of microstructure composed of a mammillary, squamatic, and external zones. -

Additional Specimen of Microraptor Provides Unique Evidence of Dinosaurs Preying on Birds
Additional specimen of Microraptor provides unique evidence of dinosaurs preying on birds Jingmai O’Connor1, Zhonghe Zhou1, and Xing Xu Key Laboratory of Evolutionary Systematics of Vertebrates, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing 100044, China Contributed by Zhonghe Zhou, October 28, 2011 (sent for review September 13, 2011) Preserved indicators of diet are extremely rare in the fossil record; The vertebral column of this specimen is complete except for even more so is unequivocal direct evidence for predator–prey its proximal and distal ends; pleurocoels are absent from the relationships. Here, we report on a unique specimen of the small thoracic vertebrae, as in dromaeosaurids and basal birds. Poor nonavian theropod Microraptor gui from the Early Cretaceous preservation prevents clear observation of sutures; however, Jehol biota, China, which has the remains of an adult enantiorni- there does not appear to be any separation between the neural thine bird preserved in its abdomen, most likely not scavenged, arches and vertebral centra, or any other indicators that the but captured and consumed by the dinosaur. We provide direct specimen is a juvenile. The number of caudal vertebrae cannot evidence for the dietary preferences of Microraptor and a nonavian be estimated, but the elongate distal caudals are tightly bounded dinosaur feeding on a bird. Further, because Jehol enantiorni- by elongated zygapophyses, as in other dromaeosaurids. The rib thines were distinctly arboreal, in contrast to their cursorial orni- cage is nearly completely preserved; both right and left sides are thurine counterparts, this fossil suggests that Microraptor hunted visible ventrally closed by the articulated gastral basket. -

Sind Vögel Dinosaurier? Eine Kritische Analyse Fossiler Befunde
W+W Special Paper B-19-4 SIND VÖGEL DINOSAURIER? EINE KRITISCHE ANALYSE FOSSILER BEFUNDE Reinhard Junker August 2019 https://www.wort-und-wissen.de/artikel/sp/b-19-4_dinos-voegel.pdf Bild: Ein rekonstruiertes und künstlerisch dargestelltes Paar von Microraptor gui (Dromaeosauridae , Micro raptorinae). (durbed.deviantart.com, CC BY-SA 3.0) Sind Vögel Dinosaurier? Inhalt 1. Einleitung ...................................................................................... 3. kompakt ..................................................................................................... 4 Methodische Vorbemerkungen ............................................................................... 5 Zitate zu schrittweisem Erwerb von Vogelmerkmalen ...................................................... 6 2. Vogelmerkmale bei Theropoden: Vorläufer oder Konvergenzen ............................................................................... 9 2.1 Federtypen und Flugfähigkeit ..................................................................... 9 2.2 Zähne und Schnabel ................................................................................ 14 Zitate zu Konvergenzen bei Zahnverlust und Ausbildung eines Schnabels ................15 2.3 Gehirn und EQ ........................................................................................ 17 2.4 Furkula ..................................................................................................... 18 2.5 Gastralia, Rippenkorb, Brustbein .............................................................. -

Poultry Through Time
is in a metastable state. The simulations also 3. Brandon, D. G. & Wald, M. Phil. Mag. 6, 1035–1044 Phys. Rev. Lett. 110, 255502 (2013). showed that the metastable domino phase (1961). 9. Kaur, I., Mishin, Y. & Gust, W. Fundamentals of Grain and 4. Sutton, A. P. & Balluffi, R. W. Interfaces in Crystalline Interphase Boundary Diffusion 3rd edn (Wiley, 1995). is stabilized when stress is applied perpen- Materials (Oxford Univ. Press, 1995). 10. Rittner, J. D. & Seidman, D. N. Phys. Rev. B 54, 6999–7015 dicularly to the plane of the simulated grain 5. Rabkin, E. I., Semenov, V. N., Shvindlerman, L. S. & (1996). boundary, so that its energy matches that of Straumal, B. B. Acta Metall. Mater. 39, 627–639 (1991). 11. Han, J., Vitek, V. & Srolovitz, D. J. Acta Mater. 104, 259–273 the stable pearl phase — thereby establishing 6. Cantwell, P. R. et al. Acta Mater. 62, 1–48 (2014). (2016). 7. Maksimova, E. L., Shvindlerman, L. S. & Straumal, B. B. 12. Watanabe, T. & Tsurekawa, S. Acta Mater. 47, 4171–4185 a true thermodynamic equilibrium between Acta Metall. 36, 1573–1583 (1988). (1999). the two phases. 8. Frolov, T., Divinski, S. V., Asta, M. & Mishin, Y. 13. Homer, E. R. Comp. Mater. Sci. 161, 244–254 (2019). Meiners and colleagues’ work clearly proves that phase transformations occur in the grain Palaeontology boundaries of pure metals, and thus opens up fresh opportunities for materials design. The number of possible polymorphs of bulk metals is generally limited, but the variety of Poultry through time grain-boundary structures and their poss- ible metastable polymorphs (sometimes Kevin Padian referred to as complexions6) is essentially boundless10,11. -

Featured Article Cranial Anatomy of Erlikosaurus Andrewsi (Dinosauria, Therizinosauria): New Insights Based on Digital Reconstru
Journal of Vertebrate Paleontology 34(6):1263–1291, November 2014 Ó 2014 by the Society of Vertebrate Paleontology FEATURED ARTICLE CRANIAL ANATOMY OF ERLIKOSAURUS ANDREWSI (DINOSAURIA, THERIZINOSAURIA): NEW INSIGHTS BASED ON DIGITAL RECONSTRUCTION STEPHAN LAUTENSCHLAGER,*,1 LAWRENCE M. WITMER,2 PERLE ALTANGEREL,3 LINDSAY E. ZANNO,4,5 and EMILY J. RAYFIELD1 1School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K., [email protected]; 2Department of Biomedical Sciences, Heritage College of Osteopathic Medicine, Ohio University, Athens, Ohio 45701, U.S.A.; 3National University of Mongolia, Ulaanbaatar, Mongolia; 4Nature Research Center, NC Museum of Natural Sciences, Raleigh, North Carolina 27695, U.S.A.; 5Department of Biology, North Carolina State University, Raleigh, North Carolina 27601, U.S.A. ABSTRACT—The skull of Erlikosaurus andrewsi from the Upper Cretaceous Baishin Tsav locality of Mongolia represents the only known three-dimensionally preserved and nearly complete skull of a therizinosaurian. Computed tomographic (CT) scanning of the original specimen and three-dimensional visualization techniques allow the cranial skeleton to be digitally prepared, disarticulated, and restored. Here, we present a detailed description of the restored skull morphology and the individual cranial elements, including visualization of the internal neurovascular and pneumatic structures. Information gained from this study is used in a revised and emended diagnosis for E. andrewsi. A reappraisal of the evolutionary and functional changes in the cranial skeleton as provided by this study supports prior proposals that a keratinous sheath or rhamphotheca was developed early in the evolution of Therizinosauria. Paralleled by the reduction of functional and replacement teeth, this development indicates a shift in the manner of food processing/procurement at the tip of the snout. -

34 Abdala.Indd
Ciencias de la Tierra y Recursos Naturales del NOA Relatorio del XX Congreso Geológico Argentino - Tucumán 2017 VERTEBRADOS FÓSILES DEL MESOZOICO DEL NOROESTE ARGENTINO Fernando ABDALA1,2, Sara BERTELLI1 1UEL, Unidad Ejecutora Lillo (CONICET-FML) Miguel Lillo 251, 4000, Tucumán, Argentina. Email: [email protected] 2Evolutionary Studies Institute, University of the Witwatersrand Johannesburgo, Sudáfrica RESUMEN En la presente contribución se detalla el registro de fósiles vertebrados mesozoicos en el noroeste de Argentina. El mismo se compone de una parte triásica y otra cretácica, pero hay también un registro puntual del Jurásico. El Triásico se presenta en la cuenca de Ischigualasto-Villa Unión en las provincias de San Juan y La Rioja y en la cuenca de Marayes-El Carrizal al sur de San Juan, mientras que el Jurásico Inferior existe en la última cuenca y en la Formación Cañón del Colorado en el centro de la provincia de San Juan. El Cretácico está documentado en la Formación Los Llanos de La Rioja y en la cuenca del Noroeste en Jujuy y Salta. El Triásico Superior es el mejor representado mostrando una buena diversidad de arcosauromorfos. El mejor registro de dinosauriformes no dinosaurianos es en la Formación Chañares, mientras que los dinosaurios más antiguos del mundo aparecen ya bien diversifi cados en la Formación Ischigualasto y se vuelven abundantes en Los Colorados. Dicinodontes son componentes residuales (poco diversos y abundantes), mientras que los cinodontes son abundantes en la Formación Chañares y abundantes y más diversifi cados en la fauna de Ischigualasto. Registros antiguos de tortugas existen en las formaciones Los Colorados y Quebrada del Barro, y en esta última hay también esfeno- dontes.