Summary Conservation Action Plans for Mongolian Fishes Public Disclosure Authorized
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
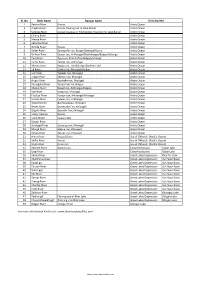
List of Rivers of Mongolia
Sl. No River Name Russian Name Draining Into 1 Yenisei River Russia Arctic Ocean 2 Angara River Russia, flowing out of Lake Baikal Arctic Ocean 3 Selenge River Сэлэнгэ мөрөн in Sükhbaatar, flowing into Lake Baikal Arctic Ocean 4 Chikoy River Arctic Ocean 5 Menza River Arctic Ocean 6 Katantsa River Arctic Ocean 7 Dzhida River Russia Arctic Ocean 8 Zelter River Зэлтэрийн гол, Bulgan/Selenge/Russia Arctic Ocean 9 Orkhon River Орхон гол, Arkhangai/Övörkhangai/Bulgan/Selenge Arctic Ocean 10 Tuul River Туул гол, Khentii/Töv/Bulgan/Selenge Arctic Ocean 11 Tamir River Тамир гол, Arkhangai Arctic Ocean 12 Kharaa River Хараа гол, Töv/Selenge/Darkhan-Uul Arctic Ocean 13 Eg River Эгийн гол, Khövsgöl/Bulgan Arctic Ocean 14 Üür River Үүрийн гол, Khövsgöl Arctic Ocean 15 Uilgan River Уйлган гол, Khövsgöl Arctic Ocean 16 Arigiin River Аригийн гол, Khövsgöl Arctic Ocean 17 Tarvagatai River Тарвагтай гол, Bulgan Arctic Ocean 18 Khanui River Хануй гол, Arkhangai/Bulgan Arctic Ocean 19 Ider River Идэр гол, Khövsgöl Arctic Ocean 20 Chuluut River Чулуут гол, Arkhangai/Khövsgöl Arctic Ocean 21 Suman River Суман гол, Arkhangai Arctic Ocean 22 Delgermörön Дэлгэрмөрөн, Khövsgöl Arctic Ocean 23 Beltes River Бэлтэсийн Гол, Khövsgöl Arctic Ocean 24 Bügsiin River Бүгсийн Гол, Khövsgöl Arctic Ocean 25 Lesser Yenisei Russia Arctic Ocean 26 Kyzyl-Khem Кызыл-Хем Arctic Ocean 27 Büsein River Arctic Ocean 28 Shishged River Шишгэд гол, Khövsgöl Arctic Ocean 29 Sharga River Шарга гол, Khövsgöl Arctic Ocean 30 Tengis River Тэнгис гол, Khövsgöl Arctic Ocean 31 Amur River Russia/China -

Monroe County Soil & Water Conservation District Fish Program
Monroe County Soil & Water Conservation District Fish Program Catalog Inside Triploid Grass Carp………………….2 Fathead Minnows…....3 Channel Catfish……...4 Koi (no longer offered).…..5 Yellow Perch………...6 Largemouth Bass..…...7 Fish Stocking Program The Monroe County Soil and Water Conservation District’s fish program is a Rainbow Trout……….8 biannual event, offered in spring and summer each year. Harsh winters cause many fish kills in ponds throughout Monroe County, and the Soil and Water Conservation District offers this program to help restock ponds. The species Black Crappie………..9 typically available for stocking include, but are not limited to: Pumpkinseed……….10 Triploid grass carp (10-12”), Catfish (6”), Fathead minnows (1-1.5”), Yellow Perch (2-3" or 4-6”) and Largemouth Bass (2-3” or 4-6”) Barley Straw……......11 You will need to bring 20 gallons of pond water for every 6 Grass Carp, 30 Fish Habitat Spheres..12 Catfish, 30 Goldfish, 500 minnows, 100 Bass, or 150 Perch for a travel time of 30-45 minutes. Please Do NOT use tap water! Use pond water and be sure to bring a cover for the container(s) you’ll be using, so the water doesn’t splash and your fish can’t escape. Five gallon pails, old coolers, trash cans or other similar containers will work. Monroe County Soil and Water Conservation District 145 Paul Road, Building #5 Rochester, NY 14624 (585) 753-7380 Monroe County Soil & Water http://www.monroecountyswcd.org/ Conservation District Providing Today, Protecting Tomorrow This document has been produced to provide a summary of all the species that MCSWCD offers Fish Catalog Page 2 Triploid Grass Carp (Ctenopharyngodon idella) Photo courtesy of New York State Department of Conservation. -

Edna Assay Development
Environmental DNA assays available for species detection via qPCR analysis at the U.S.D.A Forest Service National Genomics Center for Wildlife and Fish Conservation (NGC). Asterisks indicate the assay was designed at the NGC. This list was last updated in June 2021 and is subject to change. Please contact [email protected] with questions. Family Species Common name Ready for use? Mustelidae Martes americana, Martes caurina American and Pacific marten* Y Castoridae Castor canadensis American beaver Y Ranidae Lithobates catesbeianus American bullfrog Y Cinclidae Cinclus mexicanus American dipper* N Anguillidae Anguilla rostrata American eel Y Soricidae Sorex palustris American water shrew* N Salmonidae Oncorhynchus clarkii ssp Any cutthroat trout* N Petromyzontidae Lampetra spp. Any Lampetra* Y Salmonidae Salmonidae Any salmonid* Y Cottidae Cottidae Any sculpin* Y Salmonidae Thymallus arcticus Arctic grayling* Y Cyrenidae Corbicula fluminea Asian clam* N Salmonidae Salmo salar Atlantic Salmon Y Lymnaeidae Radix auricularia Big-eared radix* N Cyprinidae Mylopharyngodon piceus Black carp N Ictaluridae Ameiurus melas Black Bullhead* N Catostomidae Cycleptus elongatus Blue Sucker* N Cichlidae Oreochromis aureus Blue tilapia* N Catostomidae Catostomus discobolus Bluehead sucker* N Catostomidae Catostomus virescens Bluehead sucker* Y Felidae Lynx rufus Bobcat* Y Hylidae Pseudocris maculata Boreal chorus frog N Hydrocharitaceae Egeria densa Brazilian elodea N Salmonidae Salvelinus fontinalis Brook trout* Y Colubridae Boiga irregularis Brown tree snake* -

Key Sites for Key Sites for Conservation
Directory of Important Bird Areas in Mongolia: KEY SITES FOR CONSERVATION A project of In collaboration with With the support of Printing sponsored by Field surveys supported by Directory of Important Bird Areas in Mongolia: KEY SITES FOR CONSERVATION Editors: Batbayar Nyambayar and Natsagdorj Tseveenmyadag Major contributors: Ayurzana Bold Schagdarsuren Boldbaatar Axel Bräunlich Simba Chan Richard F. A. Grimmett and Andrew W. Tordoff This document is an output of the World Bank study Strengthening the Safeguard of Important Areas of Natural Habitat in North-East Asia,fi nanced by consultant trust funds from the government of Japan Ulaanbaatar, January 2009 An output of: The World Bank study Strengthening the Safeguard of Important Areas of Natural Habitat in North-East Asia,fi nanced by consultant trust funds from the government of Japan Implemented by: BirdLife International, the Wildlife Science and Conservation Center and the Institute of Biology of the Mongolian Academy of Sciences In collaboration with: Ministry of Nature, Environment and Tourism Supporting organisations: WWF Mongolia, WCS Mongolia Program and the National University of Mongolia Editors: Batbayar Nyambayar and Natsagdorj Tseveenmyadag Major contributors: Ayurzana Bold, Schagdarsuren Boldbaatar, Axel Bräunlich, Simba Chan, Richard F. A. Grimmett and Andrew W. Tordoff Maps: Dolgorjav Sanjmyatav, WWF Mongolia Cover illustrations: White-naped Crane Grus vipio, Dalmatian Pelican Pelecanus crispus, Whooper Swans Cygnus cygnus and hunters with Golden Eagles Aquila chrysaetos (Batbayar Nyambayar); Siberian Cranes Grus leucogeranus (Natsagdorj Tseveenmyadag); Saker Falcons Falco cherrug and Yellow-headed Wagtail Motacilla citreola (Gabor Papp). ISBN: 978-99929-0-752-5 Copyright: © BirdLife International 2009. All rights reserved. The use and reproduction of any part of this publication is welcomed for non-commercial purposes only, provided that the source is acknowledged Suggested citation: Nyambayar, B. -

Chanodichthys Recurviceps (A Fish, No Common Name) Ecological Risk Screening Summary
Chanodichthys recurviceps (a fish, no common name) Ecological Risk Screening Summary U.S. Fish and Wildlife Service, June 2012 Revised, November 2016 Web Version, 6/18/2018 Photo: H. T. Cheng. Licensed under CC BY-NC. Available: http://naturewatch.org.nz/taxa/187285-Culter-recurviceps. (November 2016). 1 Native Range and Status in the United States Native Range From Zhao and Cui (2011): “Known from Zhu Jiang River (Pearl River) in Guangdong and Guanxi Provinces, and Hainan Province in China.” Status in the United States This species has not been reported in the United States. 1 Means of Introductions in the United States This species has not been reported in the United States. 2 Biology and Ecology Taxonomic Hierarchy and Taxonomic Standing From ITIS (2016): “Kingdom Animalia Subkingdom Bilateria Infrakingdom Deuterostomia Phylum Chordata Subphylum Vertebrata Infraphylum Gnathostomata Superclass Osteichthyes Class Actinopterygii Subclass Neopterygii Infraclass Teleostei Superorder Ostariophysi Order Cypriniformes Superfamily Cyprinoidea Family Cyprinidae Genus Culter Basilewsky, 1855 Species Culter recurviceps (Richardson, 1846)” From Eschmeyer et al. (2016): “recurviceps, Leuciscus Richardson [J.] 1846:295 [Report of the British Association for the Advancement of Science 15th meeting [1845] […]] Canton, China. No types known. Based solely on an illustration by Reeves (see Whitehead 1970:210, Pl. 17a […]). •Valid as Erythroculter recurviceps (Richardson 1846) -- (Lu in Pan et al. 1991:93 […]). •Questionably the same as Culter alburnus Basilewsky 1855 -- (Bogutskaya & Naseka 1996:24 […], Naseka 1998:75 […]). •Valid as Culter recurviceps (Richardson 1846) -- (Luo & Chen in Chen et al. 1998:188 […], Zhang et al. 2016:59 […]). •Valid as Chanodichthys recurviceps (Richardson 1846) -- (Kottelat 2013:87 […]). -

Analysis of Genetic Diversity of Leuciscus Leuciscus Baicalensis Using Novel Microsatellite Markers with Cross-Species Transferability
Analysis of genetic diversity of Leuciscus leuciscus baicalensis using novel microsatellite markers with cross-species transferability D.J. Lei1, G. Zhao1, P. Xie1, Y. Li1, H. Yuan1, M. Zou1, J.G. Niu2 and X.F. Ma1 1College of Fisheries, Huazhong Agricultural University, Wuhan, China 2Xinjiang Fisheries Research Institute, Urumqi, China Corresponding author: X.F. Ma E-mail: [email protected] Genet. Mol. Res. 16 (2): gmr16029376 Received September 23, 2016 Accepted March 8, 2017 Published May 4, 2017 DOI http://dx.doi.org/10.4238/gmr16029376 Copyright © 2017 The Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution ShareAlike (CC BY-SA) 4.0 License. ABSTRACT. We used next-generation sequencing technology to characterize 19 genomic simple sequence repeat (SSR) markers and 11 expressed sequence tag (EST) SSR markers from Leuciscus leuciscus baicalensis, a small freshwater fish that is widely distributed in Xinjiang, China. Primers were used to test for polymorphisms in three L. leuciscus baicalensis populations in Xinjiang. There were 4-27 (average 11.3) alleles (NA), the expected heterozygosity (HE) was 0.36-0.94 (average 0.75 ± 0.14), the observed heterozygosity (HO) was 0.37-1.00 (average 0.68 ± 0.18), and the polymorphism information content (PIC) was 0.31-0.93 (average 0.71). The averages of HE and PIC for the EST-SSR markers were slightly lower than for the genomic SSR markers. Genetic analysis of the three populations showed similar results for PIC, HE, and NA. Amplifications were performed in nine other species; the top three transferability values were for Rutilus lacustris (80%), Leuciscus idus (76.7%), and Phoxinus ujmonensis (63.3%), with the following average values: PIC (0.56, 4.46, and 0.52); NA (0.40, 3.00, and 0.32); and HO (0.44, 2.74, and 0.22), respectively. -

Huchen (Hucho Hucho) ERSS
Huchen (Hucho hucho) Ecological Risk Screening Summary U.S. Fish & Wildlife Service, April 2011 Revised, January 2019, February 2019 Web Version, 4/30/2019 Photo: Liquid Art. Licensed under CC-SA 4.0 International. Available: https://commons.wikimedia.org/wiki/File:Danube_Salmon_-_Huchen_(Hucho_hucho).jpg. (January 2019). 1 Native Range and Status in the United States Native Range From Froese and Pauly (2019): “Europe: Danube drainage [Austria, Bosnia and Herzegovina, Bulgaria, Croatia, Germany, Hungary, Italy, Romania, Serbia, Slovakia, Slovenia, Switzerland, and Ukraine].” “Population has declined [in Slovenia] due to pollution and river regulation. Conservation measures include artificial propagation and stocking [Povz 1996]. Status of threat: Regionally extinct [Bianco and Ketmaier 2016].” 1 “Considered locally extinct (extirpated) in 1990 [in Switzerland] [Vilcinskas 1993].” “Extinct in the wild in 2000 [in Czech Republic] [Lusk and Hanel 2000]. This species is a native species in the basin of the Black Sea (the rivers Morava and Dyje). At present, its local and time- limited occurrence depends on the stocking material from artificial culture. Conditions that will facilitate the formation of a permanent population under natural conditions are not available [Lusk et al. 2004]. […] Status of threat: extinct in the wild [Lusk et al. 2011].” From Freyhof and Kottelat (2008): “The species is severely fragmented within the Danube drainage, where most populations exclusively depend on stocking and natural reproduction is very limited due to habitat alterations and flow regime changes.” From Grabowska et al. (2010): “The exceptional case is huchen (or Danubian salmon), Hucho hucho. The huchen’s native range in Poland was restricted to two small rivers (Czarna Orawa and Czadeczka) of the Danube River basin, […]” Status in the United States Froese and Pauly (2019) report an introduction to the United States between 1870 and 1874 that did not result in an established population. -

Beta Diversity Patterns of Fish and Conservation Implications in The
A peer-reviewed open-access journal ZooKeys 817: 73–93 (2019)Beta diversity patterns of fish and conservation implications in... 73 doi: 10.3897/zookeys.817.29337 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Beta diversity patterns of fish and conservation implications in the Luoxiao Mountains, China Jiajun Qin1,*, Xiongjun Liu2,3,*, Yang Xu1, Xiaoping Wu1,2,3, Shan Ouyang1 1 School of Life Sciences, Nanchang University, Nanchang 330031, China 2 Key Laboratory of Poyang Lake Environment and Resource Utilization, Ministry of Education, School of Environmental and Chemical Engi- neering, Nanchang University, Nanchang 330031, China 3 School of Resource, Environment and Chemical Engineering, Nanchang University, Nanchang 330031, China Corresponding author: Shan Ouyang ([email protected]); Xiaoping Wu ([email protected]) Academic editor: M.E. Bichuette | Received 27 August 2018 | Accepted 20 December 2018 | Published 15 January 2019 http://zoobank.org/9691CDA3-F24B-4CE6-BBE9-88195385A2E3 Citation: Qin J, Liu X, Xu Y, Wu X, Ouyang S (2019) Beta diversity patterns of fish and conservation implications in the Luoxiao Mountains, China. ZooKeys 817: 73–93. https://doi.org/10.3897/zookeys.817.29337 Abstract The Luoxiao Mountains play an important role in maintaining and supplementing the fish diversity of the Yangtze River Basin, which is also a biodiversity hotspot in China. However, fish biodiversity has declined rapidly in this area as the result of human activities and the consequent environmental changes. Beta diversity was a key concept for understanding the ecosystem function and biodiversity conservation. Beta diversity patterns are evaluated and important information provided for protection and management of fish biodiversity in the Luoxiao Mountains. -

Transcriptome Sequencing and Analysis of Wild Amur Ide (Leuciscus Waleckii) Inhabiting an Extreme Alkaline-Saline Lake Reveals Insights Into Stress Adaptation
Transcriptome Sequencing and Analysis of Wild Amur Ide (Leuciscus waleckii) Inhabiting an Extreme Alkaline- Saline Lake Reveals Insights into Stress Adaptation Jian Xu1, Peifeng Ji1, Baosen Wang1,2, Lan Zhao1, Jian Wang1, Zixia Zhao1, Yan Zhang1, Jiongtang Li1, Peng Xu1*, Xiaowen Sun1* 1 Centre for Applied Aquatic Genomics, Chinese Academy of Fishery Sciences, Beijing, China, 2 College of Life Sciences, Tianjin Normal University, Tianjin, China Abstract Background: Amur ide (Leuciscus waleckii) is an economically and ecologically important species in Northern Asia. The Dali Nor population inhabiting Dali Nor Lake, a typical saline-alkaline lake in Inner Mongolia, is well-known for its adaptation to extremely high alkalinity. Genome information is needed for conservation and aquaculture purposes, as well as to gain further understanding into the genetics of stress tolerance. The objective of the study is to sequence the transcriptome and obtain a well-assembled transcriptome of Amur ide. Results: The transcriptome of Amur ide was sequenced using the Illumina platform and assembled into 53,632 cDNA contigs, with an average length of 647 bp and a N50 length of 1,094 bp. A total of 19,338 unique proteins were identified, and gene ontology and KEGG (Kyoto Encyclopedia of Genes and Genomes) analyses classified all contigs into functional categories. Open Reading Frames (ORFs) were detected from 34,888 (65.1%) of contigs with an average length of 577 bp, while 9,638 full-length cDNAs were identified. Comparative analyses revealed that 31,790 (59.3%) contigs have a significant similarity to zebrafish proteins, and 27,096 (50.5%), 27,524 (51.3%) and 27,996 (52.2%) to teraodon, medaka and three- spined stickleback proteins, respectively. -

Guide 3 – Fish Farmer's Guide to Combating Parasitic
GUIDE 3 – FISH FARMER’S GUIDE TO COMBATING PARASITIC INFECTIONS IN COMMON CARP AQUACULTURE e-NIPO: 833-20-103-X A Series of ParaFishControl Guides to Combating Fish Parasite Infections in Aquaculture. Guide 3 This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 634429 (ParaFishControl). This output reflects only the author’s view and the European Union cannot be held responsible for any use that may be made of the information contained therein. Wherever the fish are, that's where we go. “ Richard Wagner “ Common carp is the third most cultivated freshwater species in the world. Carp aquaculture is usually performed in a semi-intensive manner, in earthen ponds, where parasitic diseases can easily compromise fish health, especially in the hot summer months, leading to production and economic losses. This guide provides useful information about the biological background of five parasites, their diagnostics and control measures. © A.S. Holzer List of Authors Dr Astrid S. Holzer, Principal Investigator and Team Leader Institute of Parasitology Biology Centre of the Czech Academy of Sciences, Czech Republic Email: [email protected] Dr Pavla Bartošová-Sojková, Researcher Institute of Parasitology Biology Centre of the Czech Academy of Sciences, Czech Republic Email: [email protected] Honorary Prof. Csaba Székely, Scientific Advisor and Team Leader Institute for Veterinary Medical Research, Centre for Agricultural Research, (former Hungarian Academy of Sciences), Hungary Email: [email protected] Dr Gábor Cech, Senior Researcher, Institute for Veterinary Medical Research, Centre for Agricultural Research, (former Hungarian Academy of Sciences), Hungary Email: [email protected] Dr Kálmán Molnár, Retired Scientific Advisor, Fish Pathology and Parasitology Research Team, Institute for Veterinary Medical Research, Centre for Agricultural Research (former Hungarian Academy of Sciences), Hungary Prof. -

Summary Report of Freshwater Nonindigenous Aquatic Species in U.S
Summary Report of Freshwater Nonindigenous Aquatic Species in U.S. Fish and Wildlife Service Region 4—An Update April 2013 Prepared by: Pam L. Fuller, Amy J. Benson, and Matthew J. Cannister U.S. Geological Survey Southeast Ecological Science Center Gainesville, Florida Prepared for: U.S. Fish and Wildlife Service Southeast Region Atlanta, Georgia Cover Photos: Silver Carp, Hypophthalmichthys molitrix – Auburn University Giant Applesnail, Pomacea maculata – David Knott Straightedge Crayfish, Procambarus hayi – U.S. Forest Service i Table of Contents Table of Contents ...................................................................................................................................... ii List of Figures ............................................................................................................................................ v List of Tables ............................................................................................................................................ vi INTRODUCTION ............................................................................................................................................. 1 Overview of Region 4 Introductions Since 2000 ....................................................................................... 1 Format of Species Accounts ...................................................................................................................... 2 Explanation of Maps ................................................................................................................................ -

Genomic Signature of Highland Adaptation in Fish: a Case Study in Tibetan Schizothoracinae Species Chao Tong1,2,3* , Fei Tian1 and Kai Zhao1*
Tong et al. BMC Genomics (2017) 18:948 DOI 10.1186/s12864-017-4352-8 RESEARCH ARTICLE Open Access Genomic signature of highland adaptation in fish: a case study in Tibetan Schizothoracinae species Chao Tong1,2,3* , Fei Tian1 and Kai Zhao1* Abstract Background: Genome-wide studies on highland adaptation mechanism in terrestrial animal have been widely reported with few available for aquatic animals. Tibetan Schizothoracinae species are ideal model systems to study speciation and adaptation of fish. The Schizothoracine fish, Gymnocypris przewalskii ganzihonensis had underwent the ecological niche shift from salt water to freshwater, and also experienced a recent split from Gymnocypris przewalskii przewalskii.In addition, G. p. ganzihonensis inhabited harsh aquatic environment including low temperature and hypoxia as well as other Schizothoracinae species, its genetic mechanism of highland adaptation have yet to be determined. Results: Our study used comparative genomic analysis based on the transcriptomic data of G. p. ganzihonensis and other four fish genome datasets to investigate the genetic basis of highland adaptation in Schizothoracine fish. We found that Schizothoracine fish lineage on the terminal branch had an elevated dN/ dS ratio than its ancestral branch. A total of 202 gene ontology (GO) categories involved into transport, energy metabolism and immune response had accelerated evolutionary rates than zebrafish. Interestingly, we also identified 162 genes showing signature of positive selection (PSG) involved into energy metabolism, transport and immune response in G. p. ganzihonesis. While, we failed to find any PSG related to hypoxia response as previous studies. Conclusions: Comparative genomic analysis based on G. p. ganzihonensis transcriptome data revealed significant genomic signature of accelerated evolution ongoing within Tibetan Schizothoracinae species lineage.