Clinical and Genetic Findings of Two Cases with Apert Syndrome
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cavus Foot, from Neonates to Adolescents
Orthopaedics & Traumatology: Surgery & Research (2012) 98, 813—828 Available online at www.sciencedirect.com REVIEW ARTICLE ଝ Cavus foot, from neonates to adolescents P. Wicart Paris Descartes University, Necker—Sick Children Hospital (AP—HP), 149, rue de Sèvres, Paris 75015, France Accepted: 10 July 2012 KEYWORDS Summary Pes cavus, defined as a high arch in the sagittal plane, occurs in various clinical situa- Pes cavus; tions. A cavus foot may be a variant of normal, a simple morphological characteristic, seen in Charcot-Marie-Tooth healthy individuals. Alternatively, cavus may occur as a component of a foot deformity. When it disease; is the main abnormality, direct pes cavus should be distinguished from pes cavovarus. In direct Neurology; pes cavus, the deformity occurs only in the sagittal plane (in the forefoot, hindfoot, or both). Morphological types Direct pes cavus may be related to a variety of causes, although neurological diseases predom- inate in posterior pes cavus. Pes cavovarus is a three-dimensional deformity characterized by rotation of the calcaneopedal unit (the foot minus the talus). This deformity is caused by palsy of the intrinsic foot muscles, usually related to Charcot-Marie-Tooth disease. The risk of pro- gression during childhood can be eliminated by appropriate conservative treatment (orthosis to realign the foot). Extra-articular surgery is indicated when the response to orthotic treatment is inadequate. Muscle transfers have not been proven effective. Triple arthrodesis (talocalcanear, talonavicular, and calcaneocuboid) accelerates the mid-term development of osteoarthritis in the adjacent joints and should be avoided. © 2012 Published by Elsevier Masson SAS. Introduction The cavus may be either one of the components or the main component of the deformity (Tables 1 and 2). -

Genetic, Cytogenetic and Physical Refinement of the Autosomal Recessive CMT Linked to 5Q31ð Q33: Exclusion of Candidate Genes I
European Journal of Human Genetics (1999) 7, 849–859 © 1999 Stockton Press All rights reserved 1018–4813/99 $15.00 t http://www.stockton-press.co.uk/ejhg ARTICLE Genetic, cytogenetic and physical refinement of the autosomal recessive CMT linked to 5q31–q33: exclusion of candidate genes including EGR1 Ang`ele Guilbot1, Nicole Ravis´e1, Ahmed Bouhouche6, Philippe Coullin4, Nazha Birouk6, Thierry Maisonobe3, Thierry Kuntzer7, Christophe Vial8, Djamel Grid5, Alexis Brice1,2 and Eric LeGuern1,2 1INSERM U289, 2F´ed´eration de Neurologie and 3Laboratoire de Neuropathologie R Escourolle, Hˆopital de la Salpˆetri`ere, Paris 4Laboratoire de cytog´en´etique, Villejuif 5G´en´ethon, Evry, France 6Service de Neurologie, Hˆopital des Sp´ecialit´es, Rabat, Morocco 7Service de Neurologie, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland 8Service D’EMG et de pathologie neuromusculaire, Hˆopital neurologique Pierre Wertheimer, Lyon, France Charcot-Marie-Tooth disease is an heterogeneous group of inherited peripheral motor and sensory neuropathies with several modes of inheritance: autosomal dominant, X-linked and autosomal recessive. By homozygosity mapping, we have identified, in the 5q23–q33 region, a third locus responsible for an autosomal recessive form of demyelinating CMT. Haplotype reconstruction and determination of the minimal region of homozygosity restricted the candidate region to a 4 cM interval. A physical map of the candidate region was established by screening YACs for microsatellites used for genetic analysis. Combined genetic, cytogenetic and physical mapping restricted the locus to a less than 2 Mb interval on chromosome 5q32. Seventeen consanguineous families with demyelinating ARCMT of various origins were screened for linkage to 5q31–q33. -

Tumor in Patients with 9Q22.3 Microdeletion Syndrome Suggests a Role for PTCH1 in Nephroblastomas
European Journal of Human Genetics (2013) 21, 784–787 & 2013 Macmillan Publishers Limited All rights reserved 1018-4813/13 www.nature.com/ejhg SHORT REPORT Wilms’ tumor in patients with 9q22.3 microdeletion syndrome suggests a role for PTCH1 in nephroblastomas Bertrand Isidor*,1,2,14, Franck Bourdeaut3,4,5,14, Delfine Lafon6, Ghislaine Plessis7, Elodie Lacaze7, Caroline Kannengiesser8, Sylvie Rossignol9, Olivier Pichon1, Annaig Briand1, Dominique Martin-Coignard10, Maria Piccione11, Albert David1, Olivier Delattre2,Ce´cile Jeanpierre12, Nicolas Se´venet6 and Ce´dric Le Caignec1,13 Nephroblastoma (Wilms’ tumor; WT) is the most common renal tumor of childhood. To date, several genetic abnormalities predisposing to WT have been identified in rare overgrowth syndromes. Among them, abnormal methylation of the 11p15 region, GPC3 and DIS3L2 mutations, which are responsible for Beckwith–Wiedemann, Simpson–Golabi–Behmel and Perlman syndromes, respectively. However, the underlying cause of WT remains unknown in the majority of cases. We report three unrelated patients who presented with WT in addition to a constitutional 9q22.3 microdeletion and dysmorphic/overgrowth syndrome. The size of the deletions was variable (ie, from 1.7 to 8.9 Mb) but invariably encompassed the PTCH1 gene. Subsequently, we identified a somatic PTCH1 nonsense mutation in the renal tumor of one patient. In addition, by array comparative genomic hybridization method, we analyzed the DNA extracted from the blood samples of nine patients with overgrowth syndrome and WT, but did not identify any deleterious chromosomal imbalances in these patients. These findings strongly suggest that patients with constitutional 9q22.3 microdeletion have an increased risk of WT, and that PTCH1 have a role in the pathogenesis of nephroblastomas. -

Pes Cavus – Not Just a Clinical Sign Diagnosis, Aetiology and Management
ACNRJF13_Layout 1 13/01/2013 22:33 Page 16 REHABILITATION ARTICLE Pes Cavus – Not Just a Clinical Sign Diagnosis, Aetiology and Management Mr Thomas Ball, he term Pes cavus describes the deformity of Figure 1 MRCS, MRCP, MA(Cantab) is a Specialty Registrar in Trauma a high arched, relatively stiff foot. It has a and Orthopaedics in the South T variety of neurological and other causes. West Peninsula, currently working Management depends on the aetiology, rapidity of at Royal Cornwall Hospital. He progression and the severity of symptoms. aims to specialise in foot and ankle surgery and correction of lower limb deformity. Definition Pes cavus is an umbrella term describing a spec- trum of foot shapes with a high arch.1 Pure Pes cavus occurs when the metatarsal bones are plan- tarflexed relative to the hindfoot – described as ‘forefoot plantaris’ – which increases the height and curvature of the medial longitudinal arch (Figure 1). When the patient weight-bears, the hindfoot is pushed into dorsiflexion by the plan- tarflexed forefoot (Figure 2). Mr Michael Butler, A high arch accompanied by a medially angu- MA MB BS FRCS (TR&Orth) lated heel is termed pes cavovarus (Figure 3). is a Consultant Orthopaedic Surgeon and Foot and Ankle When this is complicated by foot drop and Specialist at the Royal Cornwall equinus of the ankle, it is described as pes Hospital in Truro and is a regular equinocavovarus. A n o t h e r va r i a n t , pes calcaneo- officer in the Army. Mr Butler varus, occurs when the primary deformity is treats patients for complex excessive ankle and hindfoot dorsiflexion; in problems of the foot and ankle Box 1: Causes of Pes cavus and is a Review Board Member for order to place the foot flat on the ground, the fore- Foot and Ankle Surgery. -

A Rare Intermetatarsal Coalition with Rigid Fifth Metatarsal Deformity and Symptomatic Plantar Lesion
The Journal of Foot & Ankle Surgery xxx (2015) 1–6 Contents lists available at ScienceDirect The Journal of Foot & Ankle Surgery journal homepage: www.jfas.org Case Reports and Series A Rare Intermetatarsal Coalition With Rigid Fifth Metatarsal Deformity and Symptomatic Plantar Lesion Antonio Cordoba-Fern andez, DP, PhD 1, Rafael Rayo-Rosado, DP, PhD 2, Daniel Lopez-Garc ıa, DP 3, Jose MarıaJuarez-Jim enez, DP, PhD 2 1 Professor of Podiatric Surgery, Department of Podiatry, Universidad de Sevilla, Sevilla, Spain 2 Assistant Professor, Department of Podiatry, Universidad de Sevilla, Sevilla, Spain 3 Private Practice, Jerez de la Frontera, Spain article info abstract Level of Clinical Evidence: 4 Coalition or synostosis of the foot is a relatively uncommon abnormality. Some cases of synostosis of the foot, primarily involving the midfoot and hindfoot, have been reported. However, intermetatarsal coalition is Keywords: bone bridge extremely rare, with only a small number of cases reported. We report a case of a unilateral, congenital fi metatarsal coalition metatarsal coalition between the fourth and fth metatarsal bones in a 27-year-old female. She had initially oblique metatarsal osteotomy been referred because of a symptomatic plantar lesion under the fifth metatarsal head. Surgery consisted of synostosis separating the affected metatarsals, combined with a proximal osteotomy, which proved successful in establishing pain-free and more natural weightbearing. Ó 2015 by the American College of Foot and Ankle Surgeons. All rights reserved. Congenital coalitions of the foot are relatively uncommon abnor- malities and occur in approximately 1% of the population (1). Talo- calcaneal and calcaneonavicular are the most common types of coalitions. -

Congenital Anomalies of the Upper Extremity
I have no disclosures Provide an overview of the spectrum of congenital upper extremity anomalies Describe the key imaging findings of these abnormalities Discuss the important clinical features of these entities Not enough bones (deficiencies) Extra bones Fusion/failure of separation Malalignment Abnormal bone morphology Big bones/soft tissues Not enough bones (deficiencies) Extra bones Fusion/failure of separation Malalignment Abnormal bone morphology Big bones/soft tissues Phocomelia: deficiency/absence of the proximal-mid extremity Autosomal recessive › ESCO2 gene involved in chromosome separation Rare; 150 cases reported All extremities affected Multisystem disorder Absent radii, (near) normal thumbs Possibly more extensive upper limb and even lower limb deficiencies Autosomal recessive, 1q21.1, 1:1,000,000 Thrombocytopenia › Severe at birth; bleeding complications › Transfusion requirements decrease by age 1 › Normalization of platelet count by adulthood Radial anomaly most commonly limited to the thumb Autosomal dominant 12q24.1, 1:100,000 Cardiac manifestations › Septal defects (atrial most common) › Pulmonic stenosis Vertebral Anal Cardiac Tracheoesophageal fistula Esophageal atresia RADIAL/Renal Limb incidence 16/100,000 Tend to be associated with other non- MSK abnormalities/syndromes Goldfarb et al. (2006) › Reviewed 56 years of surgical data (Wash U) › 146 patients with radial deficiencies › 55 syndromic Goal is to preserve thumb function to be able to perform basic functions (i.e., grab objects, -

Appendix 3.1 Birth Defects Descriptions for NBDPN Core, Recommended, and Extended Conditions Updated March 2017
Appendix 3.1 Birth Defects Descriptions for NBDPN Core, Recommended, and Extended Conditions Updated March 2017 Participating members of the Birth Defects Definitions Group: Lorenzo Botto (UT) John Carey (UT) Cynthia Cassell (CDC) Tiffany Colarusso (CDC) Janet Cragan (CDC) Marcia Feldkamp (UT) Jamie Frias (CDC) Angela Lin (MA) Cara Mai (CDC) Richard Olney (CDC) Carol Stanton (CO) Csaba Siffel (GA) Table of Contents LIST OF BIRTH DEFECTS ................................................................................................................................................. I DETAILED DESCRIPTIONS OF BIRTH DEFECTS ...................................................................................................... 1 FORMAT FOR BIRTH DEFECT DESCRIPTIONS ................................................................................................................................. 1 CENTRAL NERVOUS SYSTEM ....................................................................................................................................... 2 ANENCEPHALY ........................................................................................................................................................................ 2 ENCEPHALOCELE ..................................................................................................................................................................... 3 HOLOPROSENCEPHALY............................................................................................................................................................. -

Pes Cavus: a Clinical Study with Special Reference to Its Etiology
PES CAVUS: A CLINICAL STUDY WITH SPECIAL REFERENCE TO ITS ETIOLOGY. By ESME GILROY, M.B., Ch.B. Pes Cavus has long been recognised as a distinct clinical entity, but its etiology is still a controversial subject and the theories concerning its nature and significance are so numerous as to suggest that none is in itself adequate to explain the clinical facts observed. Definition.?Pes cavus or hollow claw-foot is a deformity exhibiting three cardinal features (Steindler n). (1) An increase in the height of the longitudinal arch. (2) A dropping of the anterior arch with plantar flexion of the front of the foot. (3) A variable amount of dorsal retraction of the toes, the claw-foot deformity proper, with hyperextension of the metatarsophalangeal and flexion of the phalangeal joints. Although this paper is primarily intended as a detailed record of six cases, it was found that a proper appreciation of the points presented by these could only be attained by the analysis of a larger number. The records of a further series ?f cases have therefore been investigated ; the tabulated results are appended and form the basis for discussion. An attempt has been made to discover what condition is most frequently responsible for the deformity at different age periods, and whether there is any correlation between clinical and etiological types or a significant difference in sex or age incidence. Etiological Aspects. In 1889, F. R- Fisher3 drew attention to the prevalence of a mild degree of pes cavus and to its association with specific fevers and other acute illness. -

Biomechanical Explanations for Selective Sport Injuries of the Lower Extremity • DR
Biomechanical Explanations for Selective Sport Injuries of the Lower Extremity • DR. LEE S. COHEN • Podiatric Consultant: – Philadelphia Eagles – Philadelphia 76ers – Philadelphia Wings Understanding Normalcy What is “Normal”? Rearfoot/heel to leg Perpendicular forefoot in straight line to rearfoot Thighs and legs in straight line Understanding Normalcy Inverted Normal/Neutral Forefoot varus Heel varus or supinatus Bow-legged = Genu varum Understanding Normalcy Everted Normal/Neutral Heel valgus Forefoot valgus Knock-kneed = Genu valgum The Arches of Your Feet Rear foot High High Low (Back foot) Forefoot Arch High Low Low (Front foot) High Combo Low Arch Arch (Flatfoot) Understanding Normalcy, cont. These are abnormal foot types…a normal or neutral foot type is a happy medium between the high and low arch feet. Pes cavus = High arch foot Pes planus = Flatfoot “High-Low” = Combo foot Best Foot Forward • A person who runs or • A person who runs or walks properly: walks with a high arch: – Lands on lateral heel – Foot rolls to medial arch – Lands hard on lateral heel (pronates) while turning – Doesn’t pronate enough to inward to toe off great allow the impact of running toe to be absorbed through • A person who runs or walks flat footed: the body – Lands on lateral heel – The feet and outer part of – Foot rolls inward knee and hip bear the (pronates) excessively, brunt of each step which also causes the lower leg to turn inward excessively – With NO direct toe off Iliotibial Band Syndrome • Most common etiology of lateral knee pain in runners -

Effect of the Cavus and Planus Foot on Biomechanics Aspect of the Plantar Pressure in Adolescents with Idiopathic Scoliosis
Effect of the Cavus and Planus Foot on Biomechanics Aspect of the Plantar Pressure in Adolescents With Idiopathic Scoliosis Carlos Eduardo Gonçalves Barsotti Hospital do Servidor Publico Estadual de Sao Paulo Gustavo Alves Tostes Hospital do Servidor Publico Estadual de Sao Paulo Rodrigo Mantelatto Andrande Universidade de Sao Paulo Ariane Verttú Schmidt Universidade de Santo Amaro Alexandre Penna Torini Universidade de Santo Amaro Ana Paula Ribeiro ( [email protected] ) Universidade de São Paulo Faculdade de Medicina: Universidade de Sao Paulo Faculdade de Medicina https://orcid.org/0000-0002-1061-3789 Research Keywords: idiopathic scoliosis, adolescents, foot, plantar arch, plantar pressure Posted Date: February 15th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-229221/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/13 Abstract Purpose: To verify the effect of cavus and planus feet on plantar pressure during static posture in adolescents with idiopathic scoliosis (AIS). Methods: Cross-sectional study. Sixty adolescents with idiopathic scoliosis (AIS) were evaluated and divided into three groups: normal foot (n=20), cavus foot (n=20), and planus foot (n=20). The scoliosis was conrmed by a spine X-ray exam (Cobb angle). The plantar arch index (AI) was calculated from the ratio between the midfoot area and the total area of the foot. Distribution plantar pressure data was collected using a plantar pressure system. The contact area, maximum force, and peak pressure were acquired over areas: forefoot, midfoot, and lateral and medial rearfoot. Results: The Cobb angle of the AIS of the major curves averaged 33.7°±10.7°, the mean TK was 32.6° ±6.7°, and the mean LL was 31.4°±8.3°. -

Scoliosis Is Rapidly Progressive During the Periods of Rapid Growth In
Adolescent Idiopathic Scoliosis: Associated Factors, Progression, and a Risk-Benefit Analysis of Treatment Options Davis, Bonnie E. ABSTRACT Objective: The purpose of this paper is to discuss the associated factors of adolescent idiopathic scoliosis (AIS), the progression of AIS, and to compare conservative vs. surgical management of AIS with respect to the benefits, risks, and costs of each. After reviewing the literature, a conclusion will be made as to which type of management has the most reasonable and beneficial approach. Methods: Research literature covering AIS was obtained through colleagues, databases such as PubMed, and relevant websites. Discussion: Associated factors of AIS include female gender and genetic predispositions, respiratory deficiency, melatonin-signaling pathway deficiencies, pes cavus, and high platelet calmodulin levels. Treatment options for AIS patients include conservative care (electric stimulation, manual therapy, bracing, acupuncture), and surgical care, which may also include post-surgical bracing. Conservative care has shown some promising results for both immediate and long-lasting effects on scoliotic curvatures. Side effects are limited due to the non-invasive procedures. Surgical management of AIS can be very effective at immediate curve reduction, however long- term results may not be as promising. It also has many more serious side effects such as implant failure, infections, and decreased spinal range of motion. Conclusion: Due to numerous factors involved in the development of AIS, a holistic -
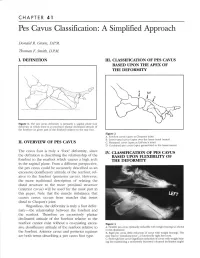
Pes Cavus Classification: a Simplified Approach
CHAPTER 41 Pes Cavus Classification: A Simplified Approach Donald R. Green, DPM. Thomas F. Smitb, D.PM. I. DEFIII-ITION III. CIASSIFICATION OF PES CAVUS BASED UPON THE APEX OF THE DEFORMITY brd (.r Figure 1. The pes cavus deformity is primarily a sagittal plane foot deformity in which there is an excessive plantar declinated attitude of the forefoot (or given part of the forefoot) relative to the rear foot. Figure 2 A. Forefoot car.us (apex at Chopafi's ioint) B. Lesser tarsal car'.us (apex over the lesser tarsal bones) II. O\rERVIEW OF PES CAVTJS C. Metatarsal car.us (apex at LisFranc's joint) D. Combined pes cavlls (apex generalized to the lesser tarsus) The cavus foot is truly a "foot" deformity, since TV. CIA.SSIFICATION OF PES CAYUS the definition is describing the relationship of the BASED UPON FLHilBILITY OF forefoot to the rearfoot which causes a high arch THE DEFORMITY in the sagittal piane. From a different perspective, the pes ca\.us could be accurately described as an excessive dorsiflexory attitude of the rearfoot, rel- ative to the forefoot (posterior cavus). However, the more traditional description of relating the distal structure to the more proximal structure (anterior cavus) will be used for the most part in this paper. Note that the muscle imbalance that causes cavus, occurs from muscles that insert distal to Chopart's joint. Regardless, the deformity is truly a foot defor- mity-the relationship between the forefoot and the rearfoot. Therefore an excessively plantar declinated attitude of the forefoot relative to the rearfoot cannot exist without a co-existing exces- Figure 3 sive dorsiflexory attitude of the rearfoot relative to A.