Aegls) for CHLOROACETYL CHLORIDE (CAS Reg. No. 79-04-9
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
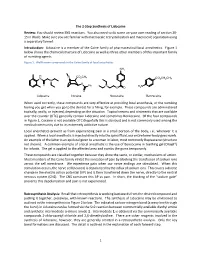
1 the 2-Step Synthesis of Lidocaine Review
The 2-Step Synthesis of Lidocaine Review: You should review SN2 reactions. You also need to do some on your own reading of section 20- 15 in Wade. Make sure you are familiar with macroscale recrystallization and macroscale separation using a separatory funnel. Introduction: Lidocaine is a member of the Caine family of pharmaceutical local anesthetics. Figure 1 below shows the chemical structure of Lidocaine as well as three other members of this important family of numbing agents. Figure 1. Well-known compounds in the Caine family of local anesthetics NH2 H N CO2CH3 N CO2CH2CH3 N O O Ph N H N O O 2 O Lidocaine Cocaine Novocaine Benzocaine When used correctly, these compounds are very effective at providing local anesthesia, or the numbing feeling you get when you go to the dentist for a filling, for example. These compounds are administered topically, orally, or injected, depending on the situation. Topical creams and ointments that are available over the counter (OTC) generally contain Lidocaine and sometimes Benzocaine. Of the four compounds in Figure 1, Cocaine is not available OTC (hopefully this is obvious) and is not commonly used among the medical community due to its extremely addictive nature. Local anesthetics prevent us from experiencing pain in a small portion of the body, i.e., wherever it is applied. When a local anesthetic is injected directly into the spinal fluid, our entire lower body goes numb. An example of the latter is an epidural given to a woman in labor, most commonly Bupivacaine (structure not shown). A common example of a local anesthetic is the use of Benzocaine in teething gel (Orajel®) for infants. -

Assessment of Portable HAZMAT Sensors for First Responders
The author(s) shown below used Federal funds provided by the U.S. Department of Justice and prepared the following final report: Document Title: Assessment of Portable HAZMAT Sensors for First Responders Author(s): Chad Huffman, Ph.D., Lars Ericson, Ph.D. Document No.: 246708 Date Received: May 2014 Award Number: 2010-IJ-CX-K024 This report has not been published by the U.S. Department of Justice. To provide better customer service, NCJRS has made this Federally- funded grant report available electronically. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. Assessment of Portable HAZMAT Sensors for First Responders DOJ Office of Justice Programs National Institute of Justice Sensor, Surveillance, and Biometric Technologies (SSBT) Center of Excellence (CoE) March 1, 2012 Submitted by ManTech Advanced Systems International 1000 Technology Drive, Suite 3310 Fairmont, West Virginia 26554 Telephone: (304) 368-4120 Fax: (304) 366-8096 Dr. Chad Huffman, Senior Scientist Dr. Lars Ericson, Director UNCLASSIFIED This project was supported by Award No. 2010-IJ-CX-K024, awarded by the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice. The opinions, findings, and conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect those of the Department of Justice. This document is a research report submitted to the U.S. Department of Justice. This report has not been published by the Department. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. -

Material Safety Data Sheet
Material Safety Data Sheet Chloroacetyl chloride, 98% ACC# 00956 Section 1 - Chemical Product and Company Identification MSDS Name: Chloroacetyl chloride, 98% Catalog Numbers: AC147290000, AC147290010, AC147290050, AC147291000, AC147292500 Synonyms: Chloracetyl chloride; Chloroacetic acid chloride. Company Identification: Acros Organics N.V. One Reagent Lane Fair Lawn, NJ 07410 For information in North America, call: 800-ACROS-01 For emergencies in the US, call CHEMTREC: 800-424-9300 Section 2 - Composition, Information on Ingredients CAS# Chemical Name Percent EINECS/ELINCS 79-04-9 Chloroacetyl chloride 98 201-171-6 Section 3 - Hazards Identification EMERGENCY OVERVIEW Appearance: colorless to light yellow liquid. Danger! Corrosive. Causes eye and skin burns. Causes digestive and respiratory tract burns. Harmful if swallowed, inhaled, or absorbed through the skin. Lachrymator (substance which increases the flow of tears). Moisture sensitive. Corrosive to metal. Target Organs: Lungs. Potential Health Effects Eye: Lachrymator (substance which increases the flow of tears). Causes eye irritation and burns. Skin: Harmful if absorbed through the skin. Causes severe skin irritation and burns. Ingestion: Harmful if swallowed. Causes gastrointestinal tract burns. Inhalation: Harmful if inhaled. Causes severe irritation of upper respiratory tract with coughing, burns, breathing difficulty, and possible coma. May cause abdominal pain, nausea, vomiting, and inflammation of the gums and mouth. Inhalation of high concentrations may cause pulmonary edema. Chronic: Prolonged or repeated exposure may cause lung irritation, chest pain, and pulmonary edema. Section 4 - First Aid Measures Eyes: In case of contact, immediately flush eyes with plenty of water for a t least 15 minutes. Get medical aid immediately. Skin: In case of contact, immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. -

Acetic Anhydride
ACETIC ANHYDRIDE Method number: 102 Matrix: Air Target concentration: 5 ppm (20 mg/m3) OSHA PEL: 5 ppm (20 mg/m3) TWA ACGIH TLV: 5 ppm (20 mg/m3) ceiling Procedure: Samples are collected open face on glass fiber filters coated with veratrylamine and di-n-octyl phthalate. Samples are extracted with 50/50 (v/v) 2-propanol/toluene and analyzed by GC using a nitrogen- phosphorus detector (NPD). Recommended air volume and sampling rate: 7.5 L at 0.5 L/min ceiling 7.5 L at 0.05 L/min TWA Reliable quantitation limit: 0.094 ppm (0.39 mg/m3) Standard error of estimate at the target concentration: 6.4% Special caution: Ketene and acetyl chloride produce the same derivative as acetic anhydride. Coated filters should be used within a month of preparation. Status of method: Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. Date: October 1993 Chemist: Yihlin Chan Organic Methods Evaluation Branch OSHA Salt Lake Technical Center Salt Lake City, UT 84165-0200 1. General Discussion 1.1 Background 1.1.1 History In OSHA Method 82, acetic anhydride is collected on a glass fiber filter impregnated with 1-(2-pyridyl)piperazine, which reacts with the anhydride to form a derivative (Ref. 5.1). Attempts at using 1-(2-pyridyl)piperazine for the derivatization of maleic, phthalic, and trimellitic anhydrides failed, however, because the resulting derivatives of these anhydrides were found to be unstable. These anhydrides were derivatized with veratrylamine instead (Refs. 5.2-5.4). -

Title the Reaction of Methyl Chloride with Carbon Monoxide Author(S
Title The reaction of methyl chloride with carbon monoxide Author(s) Osugi, Jiro; Mizukami, Tetuo Citation The Review of Physical Chemistry of Japan (1964), 34(1): 7-18 Issue Date 1964-11-05 URL http://hdl.handle.net/2433/46843 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University The Review of Physical Chemistry of Japan Vol. 34 No. 1 (1964) TftE REVIEW OF PHYSICAL CHE'HISTRY OF IAPA~, VDL. 34, No. 1, 1964 THE REACTION OF METHYL CHLORIDE WITH CARBON MONOXIDE BY ]IRD ~stlf,l ANDTETUO IVIIEUI:A]II* The reaction of methyl chloride witb carbon monoxide in the presence of pumice•anhydrous sodium borate catalyst yields mainly hydrogen chloride, methane, acetyl chloride and phosgene, and deposits carbon. Moreover, hydro• gen, methylene chloride, ethyl chloride and ethylene dichloride are obtained in a trace. From [he stand point of chemical kinetics the reaction is studied. From the initial rates of the products, the values of 13.1, I8.5, 23.2 and 27.Okcal/mole are obtained as the apparent activation energies {or the formation of acetyl chloride, phosgene, hydrogen chloride and methane respectively. Furthermore, the rates of formation of acetyl chloride and pbosgene are discussed. From the apparent rate constants, the apparent activation energies are obtained. The values are 19.0 kcal/male for acetyl chloride and 17.6 kcal/mole for phosgene. The reaction mechanism is discussed. Introduction Since a few years ago, carhop monoxide has been considered as one of the important materials for organic chemical industry, so there are some utilizations, such as s}•nthetic petroleum, Reppe's carhoxy- Iation reaction and oxo reaction etc. -

Prohibited and Restricted Chemical List
School Emergency Response Plan and Management Guide Prohibited and Restricted Chemical List PROHIBITED AND RESTRICTED CHEMICAL LIST Introduction After incidents of laboratory chemical contamination at several schools, DCPS, The American Association for the Advancement of Science (AAAS) and DC Fire and Emergency Management Services developed an aggressive program for chemical control to eliminate student and staff exposure to potential hazardous chemicals. Based upon this program, all principals are required to conduct a complete yearly inventory of all chemicals located at each school building to identify for the removal and disposal of any prohibited/banned chemicals. Prohibited chemicals are those that pose an inherent, immediate, and potentially life- threatening risk, injury, or impairment due to toxicity or other chemical properties to students, staff, or other occupants of the school. These chemicals are prohibited from use and/or storage at the school, and the school is prohibited from purchasing or accepting donations of such chemicals. Restricted chemicals are chemicals that are restricted by use and/or quantities. If restricted chemicals are present at the school, each storage location must be addressed in the school's written emergency plan. Also, plan maps must clearly denote the storage locations of these chemicals. Restricted chemicals—demonstration use only are a subclass in the Restricted chemicals list that are limited to instructor demonstration. Students may not participate in handling or preparation of restricted chemicals as part of a demonstration. If Restricted chemicals—demonstration use only are present at the school, each storage location must be addressed in the school's written emergency plan. Section 7: Appendices – October 2009 37 School Emergency Response Plan and Management Guide Prohibited and Restricted Chemical List Following is a table of chemicals that are Prohibited—banned, Restricted—academic curriculum use, and Restricted—demonstration use only. -

1. A. the First Reaction Is a Friedel-Crafts Acylation (FCA), Where the Major Product Is the Para- Isomer (60% Isolated Yield)
1. a. The first reaction is a Friedel-Crafts Acylation (FCA), where the major product is the para- isomer (60% isolated yield). The second reaction is a nitration, where the incoming electrophile (nitronium ion) is directed to the ortho position of the methoxy group. The last reaction is a Wolff-Kishner reduction that converts the acetyl group into an ethyl group. The nitro group does not react under these conditions. OCH OCH OCH OCH3 3 3 3 NO2 NO2 N2H4/KOH CH3COCl/AlCl3 H2SO4/HNO3 0 oC O CH 3 O CH3 CH3 (A) Reaction 1 (B) Reaction 2 (C) Reaction 3 (P) b. The best solvent for the FC-acylation is dichloromethane. Tetrahydrofuran is a fairly strong Lewis base, which would react and deactivate the AlCl3 catalyst. Ethanol would also react with AlCl3 and form alcoholates, which are inactive at FCA catalyst. Dichloromethane is polar enough to dissolve all three compounds but does not form adducts with AlCl3. Thus, aluminum chloride maintains its Lewis acidity. 3+ c. As discussed in lecture, AlCl3*6 H2O is not suitable as catalyst because the Al is not a strong Lewis acid anymore. In addition, larger amounts of water would destroy the acetyl chloride as well (=hydrolysis, CH3COCl + H2O ---- > CH3COOH + HCl). Consequently, the reaction would not proceed in the desired fashion. 3+ OH2 H2O OH2 Al H2O OH2 OH2 d. In order to determine the yield, one has to calculate the number of moles of the reactant and the product. nA = 1.90 mL * 0.996 g/mL/108.14 g/mol = 17.5 mmol nCH3COCl = 2.49 mL * 1.104 g/mL/78.5 g/mol = 35.0 mmol nAlCl3 = 4.67 g/133.5 g/mol = 35.0 mmol Compound (A) is the limiting reagent. -

Process for Preparing a Stabilized Chromium \III\ Propionate Solution
Europaisches Patentamt European Patent Office © Publication number: 0 194 596 Office europeen des brevets A2 © EUROPEAN PATENT APPLICATION C 07 C 53/122 © Application number: 86103020.3 ©intci.": C 07 C 51/41, C 08 L 5/00 © Date of filing: 07.03.86 C 08 L 33/26, E 21 B 47/00 © Priority: 11.03.85 US 710754 © Applicant: PHILLIPS PETROLEUM COMPANY 5th and Keeler Bartlesville Oklahoma 74004(US) © Date of publication of application: 17.09.86 Bulletin 86/38 © Inventor: Mumallah, Nairn Abdul-Kader 4519 E. Tuxedo Blvd © Designated Contracting States: Bartlesville, OK 74006IUS) AT DE FR GB IT NL SE © Inventor: Shtoyama.TodKay 5620 East Circle Bartlesville, OK 74006(US) © Representative: Dost, Wolfgang, Dr.rer.nat, Dipl.-Chem. et al. Patent- und Rechtsanwalte Bardehle-Pagenberg-Dost-Altenburg & Partner Postfach 86 0620 D-8000 Munchen 86IDE) © Process for preparing a stabilized chromium (III) propionate solution and formation treatment with a so prepared solution. © A A sulfate-free clear green solution of chromium (III) propionate is prepared by mixing aqueous propionic acid containing up to 55 weight percent propionic acid with a chromium (Vf) oxidant, such as a dichromate or chromate, and a sulfur-containing reductant, such as a bisulfite, which on reacting yields two phases: a lower sulfate-containing phase for discard or recycle and an upper phase solution of sulfate-free clear green propionate-sequestered chromium (III), the latter phase being stabilized with additional acid. The upper phase solution is useful for crosslinking polymeric viscosifiers such as partially hydrolyzed acrylamide-based polymers and the like in permeability contrast corrections in enhanced oil recovery operations. -

ETHYLENE DICHLORIDE (L,Z-Dichloroethane) April 19, 1978
NlaSM e~ 9~ 8tdtetue ~~-B~ 19tAwe3() In 1978 CONTENTS NO. TITLE DATE 19 - Z,4-DIAMINOANISOLE (4-Methoxy-m-Phenylenediamine) IN HAIR AND FUR DYES January 13, 1978 «( » 2.0 - TETRACHLOROETHYLENE (perchloroethylene) January 20, 1978 ZI - TRlMELUTIC ANHYDRIDE (TMA) February 3, 1978 ZZ - ETHYLENE THIOUREA April 11, 1978 Z3 - ETHYLENE DIBROMIDE AND DISULFIRAM TOXIC INTERACTION April 11, 1978 ( 4: 1) Z4 - DIRECT BLUE 6, DIRECT BLACK 38, DIRECT BROWN 95 Benzidine Derived Dyes April 17, 1978 Z5 - ETHYLENE DICHLORIDE (l,Z-dichloroethane) April 19, 1978 Z6 - NIAX· Catalyst ESN •••a mixture of Dimethylaminopropionitrile and Bis[Z-(dimethylamino)ethylj ether May 22,1978 27 - CHLOROETHANES: REVIEW OF TOXICITY August 21, 1978 28 - VINYL HALIDES CARCINOGENICITY Vinyl Bromide, Vinyl Chloride, Vinylidene Chloride September 21,1978 (1 _:"_ :3) 29 - GL YCIDYL ETHERS October lZ, 1978 (1 ~7 ) 30 - EPICHLOROHYDRIN October 12, 1978 ( 1~1 ) Cumulative List of NIOSH Current Intelligence Bulletins #l through ;¥o30 for 1975 through 1978 ----------.--------- U. S. DEPARTMENT OF HEALTH, EDUCATION" A /IYID WELFARE Public Health Ser"ice Center tor Disease Control National Institute tor Occupational Satety and Heallf:hiJ / / NIOSH CURRENT INTELLIGENCE BULLETIN REPRINTS - BULLETINS 19 thru 30 for 1978 Leonard J. Bah1man, Editor Technical Evaluation and Review Branch U.S. DEPARTMENT OF HEALTH, EDUCATION, AND WELFARE Public Health Service Center for Disease Control National Institute for Occupational Safety and Health Office of Extramural Coordination and Special Projects Rockville, Maryland 20857 September 1979 DISCLAIMER Mention of company names or products does not constitute endorsement by the National Institute for Occupational Safety and Health. DHEW (NIOSH) Publication No. -

United States Patent (19) (11) 4,129,595 Suzuki 45) Dec
United States Patent (19) (11) 4,129,595 Suzuki 45) Dec. 12, 1978 (54) PREPARATION OF CHLOROACETYL (56) References Cited CHLORDE PUBLICATIONS E. E. Blaise et al., Comptes Rendus (France), vol. 174, 75 Inventor: Shigeto Suzuki, San Francisco, Calif. pp. 1173-1174, (1922), (Chem. Abstr., vol. 16, 2480). 73) Assignee: Chevron Research Company, San Primary Examiner-Gerald A. Schwartz Francisco, Calif. Attorney, Agent, or Firm-D. A. Newell; John Stoner, Jr. (21) Appl. No.: 891,429 57 ABSTRACT Chloroacetyl chloride is prepared by reacting glycolic (22) Filed: Mar. 29, 1978 acid with thionyl chloride in the presence of nitrogen 51) int. C.’.............................................. CO7C 51/58 containing organic compound orphosphine compound. 52 U.S. C. ................................................ 260/544 Y 58 Field of Search .................................... 260/544 Y 3 Claims, No Drawings 4,129,595 1. 2 glycolic acid with thionyl chloride in the presence of a PREPARATION OF CHLOROACETYL CHLORDE catalytic amount of nitrogen-containing hydrocarbyl organic compound or hydrocarbyl phosphine com BACKGROUND OF THE INVENTION pound at high conversion and yield in accordance with 1. Field of the Invention 5 the present invention. The present invention relates to the preparation of chloroacetyl chloride. More particularly, the invention DETALED DESCRIPTION OF THE relates to the preparation of chloroacetyl chloride by INVENTION reacting glycolic acid with thionyl chloride in the pres The nitrogen-containing hydrocarbyl organic com ence of nitrogen-containing -

Ketene Reactions. I. the Addition of Acid Chlorides
KETENE REACTIONS. I. THE ADDITION OF ACID CHLORIDES TO DIMETHYLKETENE. II. THE CYCLOADDITION OF KETENES TO CARBONYL COMPOUNDS APPROVED: Graduate Committee: Major Professor Committee Member.rr^- Committee Member Committee Member Director of the Department of Chemistry Dean' of the Graduate School Smith, Larry, Ketene Reactions. I. The Addition of Acid Chlorides to DimethyIketene. II. The Cycloaddition of Ketenes to Carbonvl Compounds. Doctor of Philosophy (Chemistry), December, 1970, 63 pp., 3 tables, bibliography, 62 titles. Part I describes the addition of several acid chlorides to dimethylketene. The resulting 3-ketoacid chlorides were isolated and characterized. The reactivities of acid chlorides were found to parallel the parent acid pKa's. A reactivity order of ketenes toward acid chlorides was established. Dimethylketene is more reactive than ketene which is more reactive than diphenylketene. Attempts to effect the addition of an acid halide to a ketene produced by in situ dehydro- halogenation yielded a-halovinyl esters. The addition of acid chlorides to ketenes was concluded to be an ionic process dependent upon the nucleophilic character of the ketene oc- carbon and the polarity of the carbon-chlorine bond in the acid chloride. Part II describes the cycloaddition of several aldo- ketenes to chloral. The ketenes were generated in situ by dehydrohalogenation and dehalogenation of appropriately substituted acyl halides. Both cis- and trans-4-trichloro- Miyl-2-oxetanones are produced in the cycloadditions with the sterically hindered cis isomer predominating. Isomer distributions were determined by vpc or nmr analysis of the reaction solutions. Production of the ketenes by dehalo- genation resulted in enhanced reactivity of the carbonyl compounds. -

The Organic Chemistry of Drug Synthesis
THE ORGANIC CHEMISTRY OF DRUG SYNTHESIS VOLUME 3 DANIEL LEDNICER Analytical Bio-Chemistry Laboratories, Inc. Columbia, Missouri LESTER A. MITSCHER The University of Kansas School of Pharmacy Department of Medicinal Chemistry Lawrence, Kansas A WILEY-INTERSCIENCE PUBLICATION JOHN WILEY AND SONS New York • Chlchester • Brisbane * Toronto • Singapore Copyright © 1984 by John Wiley & Sons, Inc. All rights reserved. Published simultaneously in Canada. Reproduction or translation of any part of this work beyond that permitted by Section 107 or 108 of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful. Requests for permission or further information should be addressed to the Permissions Department, John Wiley & Sons, Inc. Library of Congress Cataloging In Publication Data: (Revised for volume 3) Lednicer, Daniel, 1929- The organic chemistry of drug synthesis. "A Wiley-lnterscience publication." Includes bibliographical references and index. 1. Chemistry, Pharmaceutical. 2. Drugs. 3. Chemistry, Organic—Synthesis. I. Mitscher, Lester A., joint author. II. Title. [DNLM 1. Chemistry, Organic. 2. Chemistry, Pharmaceutical. 3. Drugs—Chemical synthesis. QV 744 L473o 1977] RS403.L38 615M9 76-28387 ISBN 0-471-09250-9 (v. 3) Printed in the United States of America 10 907654321 With great pleasure we dedicate this book, too, to our wives, Beryle and Betty. The great tragedy of Science is the slaying of a beautiful hypothesis by an ugly fact. Thomas H. Huxley, "Biogenesis and Abiogenisis" Preface Ihe first volume in this series represented the launching of a trial balloon on the part of the authors. In the first place, wo were not entirely convinced that contemporary medicinal (hemistry could in fact be organized coherently on the basis of organic chemistry.