The Development of Techniques for the Evaluation and Effective Management of Surface and Groundwater Contamination in the Orange Free State Goldfields
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Resources Policy and Mine Closure in South Africa: the Case of the Free State Goldfields
Resources Policy 38 (2013) 363–372 Contents lists available at SciVerse ScienceDirect Resources Policy journal homepage: www.elsevier.com/locate/resourpol Resources policy and mine closure in South Africa: The case of the Free State Goldfields Lochner Marais n Centre for Development Support, University of the Free State, PO Box 339, Bloemfontein 9300, South Africa article info abstract Article history: There is increasing international pressure to ensure that mining development is aligned with local and Received 24 October 2012 national development objectives. In South Africa, legislation requires mining companies to produce Received in revised form Social and Labour Plans, which are aimed at addressing local developmental concerns. Against the 25 April 2013 background of the new mining legislation in South Africa, this paper evaluates attempts to address mine Accepted 25 April 2013 downscaling in the Free State Goldfields over the past two decades. The analysis shows that despite an Available online 16 July 2013 improved legislative environment, the outcomes in respect of integrated planning are disappointing, Keywords: owing mainly to a lack of trust and government incapacity to enact the new legislation. It is argued that Mining legislative changes and a national response in respect of mine downscaling are required. Communities & 2013 Elsevier Ltd. All rights reserved. Mine closure Mine downscaling Local economic development Free State Goldfields Introduction areas were also addressed. According to the new Act, mining companies are required to provide, inter alia, a Social and Labour For more than 100 years, South Africa's mining industry has Plan as a prerequisite for obtaining mining rights. These Social and been the most productive on the continent. -

MERCURY - PERSEUS 400 Kv TRANSMISSION LINE
MERCURY - PERSEUS 400 kV TRANSMISSION LINE POTENTIAL IMPACT ON THE SOIL-LANDFORM RESOURCES Prepared by: Dr EBEN VERSTER (Pr Sci Nat) PEDOPLAN CC 247 KNYSNA AVE 0182 SINOVILLE Prepared for: STRATEGIC ENVIRONMENTAL FOCUS P.O. Box 74785 Lynnwood Ridge 0040 MARCH 2003 EXECUTIVE SUMMARY 1. This report deals with a desk-top study of the soil-landform resources of the Mercury-Perseus Transmission Line Project with the objective to provide a description of these resources, interpret these features in terms of their suitability for selected land uses, identify the impact landform would have on the project, identify sensitive soils along the proposed corridors, evaluate the impacts on the soils and recommend mitigatory measures and actions to minimise negative impacts. 2. As information base, the land type maps (scale 1:250 000) and accompanying memoirs covering the survey area were used. Aspects on the soils, landform and climate were derived from the land type survey, whereas topographic and other geomorphological data were obtained from the 1:50 000 topographical maps. 3. It must be clearly stated that the land type information is for broad-scale assessment purposes only and that the positioning of pylons and construction camps, for example, will require studies at the detailed level. 4. The land type map (FIGURE 1) shows the spatial distribution of the land types, while TABLE 4.1 summarises the soil and slope components occupying each land type. 4.1 In general, five broad soil-landform systems related to parent material and topography can be distinguished. These are freely drained, red and yellow-brown, fine sandy soils on level topography; fine sandy to loamy soils in plinthic catena on level topography; duplex soils with reddish subsoils on level topography; duplex and other clayey soils of mainly bottomland sites; and very shallow and rocky soils overlying calcrete and hard rock associated with steeper slopes in places. -

An Assessment of Fish and Fisheries in Impoundments in the Upper Orange-Senqu River Basin and Lower Vaal River Basin
AN ASSESSMENT OF FISH AND FISHERIES IN IMPOUNDMENTS IN THE UPPER ORANGE-SENQU RIVER BASIN AND LOWER VAAL RIVER BASIN Submitted in fulfillment of the requirements in respect of the Doctoral Degree DOCTOR OF PHILOSOPHY in the Department of Zoology and Entomology in the Faculty of Natural and Agricultural Sciences at the University of the Free State by LEON MARTIN BARKHUIZEN 1 July 2015 Promoters: Prof. O.L.F. Weyl and Prof. J.G. van As Table of contents Abstract ........................................................................................................................................ vi Acknowledgements .............................................................................................................................. ix List of tables ....................................................................................................................................... xii List of figures ....................................................................................................................................... xv List of some acronyms used in text .................................................................................................. xviii Chapter 1 General introduction and thesis outline ...................................................................... 1 Chapter 2 General Literature Review ........................................................................................... 7 2.1 Introduction ............................................................................................................................ -

Heritage Report-Paul Roux
Phase 1 Heritage Impact Assessment for proposed new 1.5 km-long underground sewerage pipeline in Paul Roux, Thabo Mofutsanyane District Municipality, Free State Province. Report prepared by Paleo Field Services PO Box 38806, Langenhovenpark 9330 16 / 02 / 2020 Summary A heritage impact assessment was carried for a proposed new 1.5 km-long underground sewerage pipeline in Paul Roux in the Thabo Mofutsanyane District Municipality, Free State Province. The study area is situated on the farm Farm Mary Ann 712, next to the N5 national road covering a section of the Sand River floodplain which is located on the eastern outskirts of Paul Roux . The proposed footprint is underlain by well-developed alluvial and geologically recent overbank sediments of the Sand River. Investigation of exposed alluvial cuttings next to the bridge crossing shows little evidence of intact Quaternary fossil remains. Potentially fossil-bearing Tarkastad Subgroup and younger Molteno Formation strata are exposed to the southwest of the study area. These outcrops will not be impacted by the proposed development. There are no major palaeontological grounds to suspend the proposed development. The study area consists for the most part of open grassland currently used for cattle grazing. The foot survey revealed little evidence of in situ Stone Age archaeological material, capped or distributed as surface scatters on the landscape. There are also no indications of rock art, prehistoric structures or other historical structures or buildings older than 60 years within the vicinity of the study area. A large cemetery is located directly west of the proposed footprint. The modern bridge construction at the Sand River crossing is not considered to be of historical significance. -

Monthly Report on Livestock Disease Trends As Informally Reported By
Monthly report on livestock disease trends as informally reported by veterinarians belonging to the Ruminant Veterinary Association of South Africa (RuVASA), a group of the South African Veterinary Association November 2014 The following practices and la boratories (104 ) submitted reports in November 2014: Mpumalanga (10 ) Balfour – dr. Louis van Jaarsveld Bethal – dr. Hardus Pieters Delmas – dr. Johan Jooste Grootvlei – dr. Neels van Wyk Lydenburg – drs. Trümpelmann and Steyn Nelspruit – dr. André Beyte ll Middelburg – drs. Fourie and Rabie Piet Retief - drs. Niebuhr and Weber Standerton – dr. Kobie Kroon Volksrust – dr. André Visser Gauteng (5 ) Bapsfontein – dr. Evert Olivier Bronkhorstspruit – drs. De Bruin, De Bruin, Rudolph and Slabber Nigel – dr. Cindy van der Westhuizen Onderstepoort Veterinary Academic Hospital – proff. Annandale, Prozesky, Shakespeare, drs. Blignaut, Carrington, Gratwick, Grobler, Harmse, Holm and O’Dell Pretoria – dr. Hanneke Pienaar Limpopo (6 ) Bela - Bela – drs. Du Toit, Hans en, Bester and Herbst Lephalale (Ellisras) – dr. Brigitte Luck Machado (Louis Trichardt) – drs. Harris, Klopper and Jacobs Mokopane (Potgietersrus) - dr. Henk Visser Polokwane (Pietersburg) – drs. Watson, Viljoen, J ansen Van Vuuren, Van Rooyen, Snyman a nd Cremona Vaalwater - dr. Hampie van Staden North West (7 ) Brits – drs. Boshoff and Coertze Christiana - dr. Pieter Nel Leeudoringstad - dr. Ian Jonker Rustenburg – drs. Gaigher, Grobler, Sparks, Van Egdom, Van Rooyen, Goosen and Van Rensburg Schweizer - Reneke - dr. N.J. Heyns Stella - dr. Magdaleen Vosser Ventersdorp – dr. Olof Marais Free State (21 ) Bloemfontein - dr. Stephan Wessels Bothaville – dr. Johan Blaauw Bultfontein – dr. Santjie Pieterse Clocolan – drs. Marwick and Wasserman Dewetsdorp – dr. Marike Badenhorst Ficksburg – dr. Woody Kotze Frankfort - drs. Lessing, Cilliers and Janse van Rensburg Harrismith - drs. -

Early History of South Africa
THE EARLY HISTORY OF SOUTH AFRICA EVOLUTION OF AFRICAN SOCIETIES . .3 SOUTH AFRICA: THE EARLY INHABITANTS . .5 THE KHOISAN . .6 The San (Bushmen) . .6 The Khoikhoi (Hottentots) . .8 BLACK SETTLEMENT . .9 THE NGUNI . .9 The Xhosa . .10 The Zulu . .11 The Ndebele . .12 The Swazi . .13 THE SOTHO . .13 The Western Sotho . .14 The Southern Sotho . .14 The Northern Sotho (Bapedi) . .14 THE VENDA . .15 THE MASHANGANA-TSONGA . .15 THE MFECANE/DIFAQANE (Total war) Dingiswayo . .16 Shaka . .16 Dingane . .18 Mzilikazi . .19 Soshangane . .20 Mmantatise . .21 Sikonyela . .21 Moshweshwe . .22 Consequences of the Mfecane/Difaqane . .23 Page 1 EUROPEAN INTERESTS The Portuguese . .24 The British . .24 The Dutch . .25 The French . .25 THE SLAVES . .22 THE TREKBOERS (MIGRATING FARMERS) . .27 EUROPEAN OCCUPATIONS OF THE CAPE British Occupation (1795 - 1803) . .29 Batavian rule 1803 - 1806 . .29 Second British Occupation: 1806 . .31 British Governors . .32 Slagtersnek Rebellion . .32 The British Settlers 1820 . .32 THE GREAT TREK Causes of the Great Trek . .34 Different Trek groups . .35 Trichardt and Van Rensburg . .35 Andries Hendrik Potgieter . .35 Gerrit Maritz . .36 Piet Retief . .36 Piet Uys . .36 Voortrekkers in Zululand and Natal . .37 Voortrekker settlement in the Transvaal . .38 Voortrekker settlement in the Orange Free State . .39 THE DISCOVERY OF DIAMONDS AND GOLD . .41 Page 2 EVOLUTION OF AFRICAN SOCIETIES Humankind had its earliest origins in Africa The introduction of iron changed the African and the story of life in South Africa has continent irrevocably and was a large step proven to be a micro-study of life on the forwards in the development of the people. -

Provincial Gazette Provinsiale Koerant
Provincial Provinsiale Gazette Koerant Free State Province Provinsie Vrystaat Published by Authority Uitgegee op Gesag NO. 48 FRIDAY, 02 AUGUST 2019 NR.48 VRYDAG, 02 AUGUSTUS 2019 PROVINCIAL NOTICES 72 Nketoana Local Municipality: 72 Nketoana Plaaslike Munisipaliteit: Notice of 2019/20120 Municipal Tariffs................. 2 Kennisgewing van 2019/20120 Munisipale Tariewe...... 2 GENERAL NOTICES ALGEMENE KENNISGEWINGS 69 Mangaung Municipal Land Use Planning By-Law: 69 Mangaung Munisipale Grongebruik-Beplanning By- Erf 13440 Bloemfontein (Oranjesig)................... 2 Wet: Erf 13440 Bloemfontein (Oranjesig)................... 2 70 Removal of Restrictive Condition of Title: 70 Ophef van Beperkende Voorwaarde uit Akte: (A) Portion 1 of Erf 133 Langenhoven Park, (A) Gedeelte 1 van Erf 133 Langenhoven Park, District Bloemfontein Distrik Bloemfontein (B) Plot 8 Pantydefaid Small Holdings, District (B) Plot 8 Pantydefaid Small Holdings, Distrik Bloemfontein... ... ... ... ... ... ... ... ... ... ... ... ... .... 3 Bloemfontein................................................. 3 71 Mangaung Municipal Land Use Planning By-Law: 71 Mangaung Munisipale Grondgebruik-Beplanning By Erf 3446, 67 Genl. Dan Pienaar Drive, Wet: Erf 3446, 67 Genl. Dan Pienaar Drive, Bloemfontein... ... ... ... ... ... ... ... ... ... ... ... ... ........ ..... 3 Bloemfontein........................................................ 3 72 Phumelela By-Law on Municipal Land Use Planning, 2017: Vrede: Rezoning: Erven 1115, 1117 and 1118 .............................................. -

Uys Van Heerden
FREE DECEMBER 2016 • JANUARY 2017 SENWES EQUIPMENT AND JOHN DEERE’S GATOR WINNER Senwes Equipment and JCB – A perfect match Win with Nation in Conversation FOCUS ON Welkom & Odendaalsrus An introduction to OneAgri CHRISTMAS PERFORMANCE AND PRODUCTIVITY. SPECIAL OFFERS REDUCED RATES POSTPONED INSTALMENTS *Terms and conditions apply My choice, my Senwes. At Senwes Equipment we know what our clients want and we make sure that they get it. As the exclusive John Deere dealer we guarantee your access to the latest developments. PLEASEWe ensure CONTACTthat you have YOUR the best that money has to oer. NEAREST SENWES EQUIPMENT BRANCH FOR MORE INFO. CONTENTS ••••••• 8 31 4 CONTENTS EDITOR'S LETTER 20 Management of variables in a 2 It is raining! farming system with the assistance ON THE COVER PAGE of precision farming WHAT A YEAR 2016 has been for GENERAL 23 Meet Estelle ‘Senwes’ the newly established trademark of 3 Cartoon: Pieter & Tshepo 25 Herman is your Senwes Man – Senwes Equipment! With prize upon 3 Heard all over A grain marketing advisor with the prize they really created a Christmas 15 Christmas messages full package spirit. 26 How the handling of grain On the front page we have Gert MAIN ARTICLE changed over time? Part I 4 JCB and Senwes Equipment: A (Gerlu) Roos from Wonderfontein, 28 Branch manager Potchefstroom who won the second leg of the WIN perfect match! – Ruan brings colour to Potch BIG with Senwes Equipment and John Deere competition in Novem- 30 Utilise our Digital Platforms AREA FOCUS ber. In the process he won a John in an optimal manner 8 Welkom and Odendaalsrus Deere Gator 855D to the value of – In Circles! 46 Gert Roos wins R230 000 John R230 000, a wonderful gift, just in Deere Gator 855D time for Christmas. -
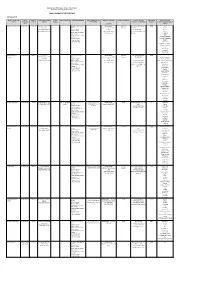
Public Libraries in the Free State
Department of Sport, Arts, Culture & Recreation Directorate Library and Archive Services PUBLIC LIBRARIES IN THE FREE STATE MOTHEO DISTRICT NAME OF FRONTLINE TYPE OF LEVEL OF TOWN/STREET/STREET STAND GPS COORDINATES SERVICES RENDERED SPECIAL SERVICES AND SERVICE STANDARDS POPULATION SERVED CONTACT DETAILS REGISTERED PERIODICALS AND OFFICE FRONTLINE SERVICE NUMBER NUMBER PROGRAMMES CENTER/OFFICE MANAGER MEMBERS NEWSPAPERS AVAILABLE IN OFFICE LIBRARY: (CHARTER) Bainsvlei Public Library Public Library Library Boerneef Street, P O Information and Reference Library hours: 446 142 Ms K Niewoudt Tel: (051) 5525 Car SA Box 37352, Services Ma-Tue, Thu-Fri: 10:00- (Metro) 446-3180 Fair Lady LANGENHOVENPARK, Outreach Services 17:00 Fax: (051) 446-1997 Finesse BLOEMFONTEIN, 9330 Electronic Books Wed: 10:00-18:00 karien.nieuwoudt@mangau Hoezit Government Info Services Sat: 8:30-12:00 ng.co.za Huisgenoot Study Facilities Prescribed books of tertiary Idees Institutions Landbouweekblad Computer Services: National Geographic Internet Access Rapport Word Processing Rooi Rose SA Garden and Home SA Sports Illustrated Sarie The New Age Volksblad Your Family Bloemfontein City Public Library Library c/o 64 Charles Information and Reference Library hours: 443 142 Ms Mpumie Mnyanda 6489 Library Street/West Burger St, P Services Ma-Tue, Thu-Fri: 10:00- (Metro) 051 405 8583 Africa Geographic O Box 1029, Outreach Services 17:00 Architect and Builder BLOEMFONTEIN, 9300 Electronic Books Wed: 10:00-18:00 Tel: (051) 405-8583 Better Homes and Garden n Government Info -

Review of Existing Infrastructure in the Orange River Catchment
Study Name: Orange River Integrated Water Resources Management Plan Report Title: Review of Existing Infrastructure in the Orange River Catchment Submitted By: WRP Consulting Engineers, Jeffares and Green, Sechaba Consulting, WCE Pty Ltd, Water Surveys Botswana (Pty) Ltd Authors: A Jeleni, H Mare Date of Issue: November 2007 Distribution: Botswana: DWA: 2 copies (Katai, Setloboko) Lesotho: Commissioner of Water: 2 copies (Ramosoeu, Nthathakane) Namibia: MAWRD: 2 copies (Amakali) South Africa: DWAF: 2 copies (Pyke, van Niekerk) GTZ: 2 copies (Vogel, Mpho) Reports: Review of Existing Infrastructure in the Orange River Catchment Review of Surface Hydrology in the Orange River Catchment Flood Management Evaluation of the Orange River Review of Groundwater Resources in the Orange River Catchment Environmental Considerations Pertaining to the Orange River Summary of Water Requirements from the Orange River Water Quality in the Orange River Demographic and Economic Activity in the four Orange Basin States Current Analytical Methods and Technical Capacity of the four Orange Basin States Institutional Structures in the four Orange Basin States Legislation and Legal Issues Surrounding the Orange River Catchment Summary Report TABLE OF CONTENTS 1 INTRODUCTION ..................................................................................................................... 6 1.1 General ......................................................................................................................... 6 1.2 Objective of the study ................................................................................................ -

South Africa)
FREE STATE PROFILE (South Africa) Lochner Marais University of the Free State Bloemfontein, SA OECD Roundtable on Higher Education in Regional and City Development, 16 September 2010 [email protected] 1 Map 4.7: Areas with development potential in the Free State, 2006 Mining SASOLBURG Location PARYS DENEYSVILLE ORANJEVILLE VREDEFORT VILLIERS FREE STATE PROVINCIAL GOVERNMENT VILJOENSKROON KOPPIES CORNELIA HEILBRON FRANKFORT BOTHAVILLE Legend VREDE Towns EDENVILLE TWEELING Limited Combined Potential KROONSTAD Int PETRUS STEYN MEMEL ALLANRIDGE REITZ Below Average Combined Potential HOOPSTAD WESSELSBRON WARDEN ODENDAALSRUS Agric LINDLEY STEYNSRUST Above Average Combined Potential WELKOM HENNENMAN ARLINGTON VENTERSBURG HERTZOGVILLE VIRGINIA High Combined Potential BETHLEHEM Local municipality BULTFONTEIN HARRISMITH THEUNISSEN PAUL ROUX KESTELL SENEKAL PovertyLimited Combined Potential WINBURG ROSENDAL CLARENS PHUTHADITJHABA BOSHOF Below Average Combined Potential FOURIESBURG DEALESVILLE BRANDFORT MARQUARD nodeAbove Average Combined Potential SOUTPAN VERKEERDEVLEI FICKSBURG High Combined Potential CLOCOLAN EXCELSIOR JACOBSDAL PETRUSBURG BLOEMFONTEIN THABA NCHU LADYBRAND LOCALITY PLAN TWEESPRUIT Economic BOTSHABELO THABA PATSHOA KOFFIEFONTEIN OPPERMANSDORP Power HOBHOUSE DEWETSDORP REDDERSBURG EDENBURG WEPENER LUCKHOFF FAURESMITH houses JAGERSFONTEIN VAN STADENSRUST TROMPSBURG SMITHFIELD DEPARTMENT LOCAL GOVERNMENT & HOUSING PHILIPPOLIS SPRINGFONTEIN Arid SPATIAL PLANNING DIRECTORATE ZASTRON SPATIAL INFORMATION SERVICES ROUXVILLE BETHULIE -

1 Title&Acknowledgements
University of Pretoria etd – Basson, H M (2005) THE ICONICITY AND LEARNABILITY OF SELECTED PICTURE COMMUNICATION SYMBOLS: A STUDY ON AFRIKAANS-SPEAKING CHILDREN by HESTER MAGDALENA BASSON In partial fulfillment of the requirements for the MASTERS DEGREE IN ALTERNATIVE AND AUGMENTATIVE COMMUNICATION Centre for AAC Faculty of Humanities UNIVERSITY OF PRETORIA Pretoria August 2004 University of Pretoria etd – Basson, H M (2005) In loving memory of WILLEM BASSON, (14/06/1984 – 20/08/2002) who taught me to live a day at a time. Met liefdevolle herinneringe aan WILLEM BASSON, (14/06/1984 – 20/08/2002) by wie ek geleer het om een dag op ‘n slag te leef. University of Pretoria etd – Basson, H M (2005) SOLI DEO GLORIA! I would like to thank the following people 1 God for giving me the strength to carry on through three difficult years. 2 Professor Erna Alant and Munyane Mophosho (who was initially involved) for all their support, ideas and patience. 3 The other staff at CAAC for their interest and encouragement. 4 Ms. R. Owen and Dr L. Louw for their help with the statistics. 5 My class, especially Amelia and Michal, who were always willing to listen. 6 Mom and Dad, for your support, patience and prayers and just being there for me. 7 Members of O9 and O12 Cell-groups for your prayers and encouragement. 8 Jaco, for helping with the finishing touches. 9 Gran Hettie, Wim, Tannie Chrissie and Tannie Dolly for all your prayers. 10 All the Myburghs for opening your house to me and allowing me to monopolize your computer.