Features and Management of the Pelvic Cancer Pain
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Use of Inflated Foley Catheters to Prevent Early Empty Pelvis Complications Following Pelvic Exenteration
ANTICANCER RESEARCH 35: 5543-5546 (2015) Use of Inflated Foley Catheters to Prevent Early Empty Pelvis Complications Following Pelvic Exenteration NICOLAE BACALBASA1, DANA TOMESCU2 and IRINA BALESCU3 1Carol Davila University of Medicine and Pharmacy, Bucharest, Romania; 2Fundeni Clinical Institute, Department of Anaethesia and Critical Care III, Bucharest, Romania; 3Ponderas Hospital, Bucharest, Romania Abstract. For most patients with bulky pelvic tumors, neoadjuvant chemo-irradiation is performed in order to pelvic exenteration remains the only curative option. diminish the local invasion and to transform the patient into Although initially reported as a palliative procedure, a candidate for a less extended resection. However, there nowadays it is rather performed with curative intent. Once are cases in which local invasion persists after neoadjuvant the resectional phase is ended, a large defect will remain at treatment and in which pelvic exenteration is needed. Since the level of the pelvic diaphragm, predisposing to severe Brunschwig reported it for the first time in 1948, this complications which are generically included under the surgical procedure has become the golden-standard for name of empty pelvis syndrome. It has been widely patients with locally invasive pelvic malignancies (3). demonstrated that this type of complication is associated Although the resectional phase has remained practically with severe mortality, even if the patient is free of any pelvic unchanged, the reconstructive phase has undergone multiple recurrence. We present the case of a 56-year-old patient improvements in order to improve the patient quality of submitted to total pelvic exenteration for locally invasive life. However, there are still cases in which a reconstruction previously chemo-irradiated cervical cancer who presented is not possible at the time of resection; in all such cases, a six months after surgery with a severe enteroperineal fistula. -
Detail Report
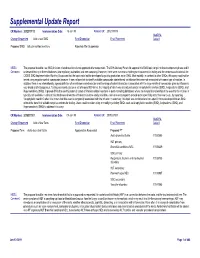
Supplemental Update Report CR Number: 2012319113 Implementation Date: 16-Jan-19 Related CR: 2012319113 MedDRA Change Requested Add a new SMQ Final Disposition Final Placement Code # Proposed SMQ Infusion related reactions Rejected After Suspension MSSO The proposal to add a new SMQ Infusion related reactions is not approved after suspension. The ICH Advisory Panel did approve this SMQ topic to go into the development phase and it Comment: underwent testing in three databases (two regulatory authorities and one company). However, there were numerous challenges encountered in testing and the consensus decision of the CIOMS SMQ Implementation Working Group was that the topic could not be developed to go into production as an SMQ. Most notably, in contrast to other SMQs, this query could not be tested using negative control compounds because it was not possible to identify suitable compounds administered via infusion that were not associated with some type of reaction. In addition, there is no internationally agreed definition of an infusion related reaction and the range of potential reactions associated with the large variety of compounds given by infusion is very broad and heterogenous. Testing was conducted on a set of around 500 terms, the majority of which was already included in Anaphylactic reaction (SMQ), Angioedema (SMQ), and Hypersensitivity (SMQ). It proved difficult to identify potential cases of infusion related reactions in post-marketing databases where the temporal relationship of the event to the infusion is typically not available. In clinical trial databases where this information is more easily available, users are encouraged to provide more specificity about the event, e.g., by reporting “Anaphylactic reaction” when it is known that this event is temporally associated with the infusion. -

Recognizing Common and Uncommon Birthmarks Harper N
Doctor should I be worried? Recognizing common and uncommon birthmarks Harper N. Price, MD, FAAD, FAAP Division Chief, Fellowship Director Friday, June 28, 10:50-11:35am Conflicts of interest: • None Learning objectives • Recognize common and less common congenital skin lesions in the outpatient setting • RED Vascular lesions: capillary malformations, hemangiomas, vascular tumors • BROWN Pigmented lesions: congenital nevi • BLUE dermal melanoyctosis • YELLOW/TAN Benign hamartomas: nevus sebaceous, connective tissue nevi • Developmental anomalies: aplasia cutis, hair collar sign • Identify those congenital skin lesions that require urgent referral and additional investigations Red birthmarks Classification of vascular anomalies • Incorrect nomenclature misunderstanding between colleagues and with patients • Incorrect nomenclature misdiagnosis • Accurate diagnosis is crucial for appropriate evaluation and management • Classification serves as a guide for clinicians Archaic terms • “Strawberry hemangioma” • “Cavernous hemangioma” • “Capillary hemangioma” • Historically speaking “hemangioma” has been used for vascular tumors and malformations Wassef M et al. Pediatr 2015 A simpler version Puttgen KB. Pediatr Clin N Am. 2014 Infantile hemangiomas (IH): classic vascular “tumor” • 4-10% of infants, head and neck • Most common soft tissue tumor of infancy • Present first few weeks of life • Proliferation of benign endothelial cells • Initial rapid growth followed by slow involution Infantile hemangiomas: risk factors • Low birth weight infants -

MRI Findings Common to Infantile Hemangiomas
MRI Findings Common to Infantile Hemangiomas Item Type text; Electronic Thesis Authors Patel, Nirav Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the College of Medicine - Phoenix, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 23/09/2021 18:48:55 Link to Item http://hdl.handle.net/10150/221633 MRI Findings Common to Infantile Hemangiomas Thesis submitted to the University of Arizona College of Medicine - Phoenix in partial fulfillment of the requirements for the degree of Doctor of Medicine Nirav Patel Class of 2012 Mentors: Judith O’Haver, PhD, RN, CPNP; Harper Price, MD; Richard Towbin, MD Dedication To my wonderful mentors Judith O’Haver, Richard Towbin, Ronald Hansen, and Harper Price. 2 Acknowledgements I would like to thank the Dermatology and Radiology departments at Phoenix Children’s Hospital for all their contributions. 3 Abstract Background: Infantile hemangiomas (IH) are the most common vascular tumors of infancy. Children may have Magnetic Resonance Imaging (MRI) to establish or confirm the diagnosis or to further evaluate lesions that do not improve with treatment. Objective: Describe specific MRI findings common to infantile hemangiomas. Compare the imaging diagnosis with the clinical diagnosis of IH to determine diagnostic accuracy. Methods: A descriptive retrospective chart review on a convenience sample. Twenty-six patients had a total of 31 MR studies in the group. From these 31 studies, 16 also had magnetic resonance angiography (MRA). -

Texas Children's Hospital Dermatology Service PCP Referral Guidelines- Vascular Birthmarks
Texas Children's Hospital Dermatology Service PCP Referral Guidelines- Vascular Birthmarks Diagnosis: VASCULAR BIRTHMARKS INFANTILE HEMANGIOMAS GENERAL INFORMATION: • Infantile hemangiomas have a natural proliferative phase, when there is rapid growth and thickening. Superficial hemangiomas (“strawberry” hemangiomas) typically experience the majority of growth in the first 8 weeks of life. Deep hemangioma may have a longer proliferative phase that can last up to 6 months. • After the first year of life, most hemangiomas are well underway in the involutional phase. The majority of hemangiomas have undergone nearly complete resolution by 4-6 years of age. REFERRAL RECOMMENDATIONS: • Please refer all hemangiomas for topical or systemic therapy may be beneficial or if there is significant parental anxiety • The following hemangioma warrant expedited appointments. If you feel your patient needs to be evaluated within 1 week, please contact the on-call dermatologist through the TCH page operator. o Ulcerated (see page 2 for recommendations while awaiting dermatology evaluation) o Possible functional compromise (e.g. eyelid, beard distribution) o High risk for cosmetic sequelae (e.g. nasal tip hemangioma, large hemangiomas with tapered base) o Large segmental hemangioma on face or lower body warranting imaging to rule-out PHACES or LUMBAR/PELVIS syndrome. o >6 Hemangiomas (please consider obtaining an abdominal ultrasound to rule out hepatic involvement prior to referral) CAPILLARY MALFORMATION (PORT-WINE STAINS) GENERAL INFORMATION: • Capillary -

Gait and Posture – Assessment in General Practice
THEME Musculoskeletal medicine Gait and posture Assessment in general practice BACKGROUND A basic analysis of a patient’s gait and posture provides information about the body and the capability of the musculoskeletal system to adjust to physical stressors. An understanding of normal gait and posture is essential for Kent Sweeting identifying and treating musculoskeletal pain. BHlthSc(Pod)(Hons), MAPodA, OBJECTIVE is a podiatrist, Brisbane, and a This article discusses normal gait and how to assess gait. It also outlines common musculoskeletal conditions and their researcher, Griffith University, Queensland. k.sweeting@ association with abnormal gait and posture. General practitioners can detect faulty postural syndromes and abnormal griffith.edu.au gait by visual scanning and awareness of pain referral patterns. Michael Mock DISCUSSION MBBS, MMed(PhysMed), Awareness of pain that can arise from faulty gait and posture will assist GPs to shift their focus away from structural FRACGP, FACPM, is a general diagnoses and unhelpful radiological investigations. The GP can become an effective facilitator of the prevention and practitioner, Wetherill Park rehabilitation of pain problems where abnormal gait and posture are found to be a main contributing factor. and St Leonards, New South Wales. Gait analysis is comparable to an X-ray or blood test; 40% respectively.5 Figure 1 summarises all events and it is a powerful investigative tool, which together with the timing of just over one complete gait cycle. When the patient history and physical examination, may examining gait, clinicians can fall into the trap of solely be used to assess and diagnose patients suffering focusing on the stance phase and miss vital pieces of musculoskeletal pain, and predict successful treatment information that can be gathered from the patient’s swing of these pathologies.1–3 Understanding the basic phase (eg. -

Role of Imaging in Gynecologic Oncology and Advanced Pelvic Surgery
Role of imaging in gynecologic oncology and advanced pelvic surgery Poster No.: C-1033 Congress: ECR 2012 Type: Educational Exhibit Authors: E. Alvarez Moreno1, J. J. Gómez Herrera2, M. Jimenez De La Peña3, R. Sainz de la Cuesta1, R. Cano Alonso4, L. Herraiz Hidalgo5; 1Pozuelo de Alarcón/ES, 2POZUELO DE ALARCON. MADRID, Spain/ES, 3Pozuelo de Alarcón, Madrid/ES, 4POZUELO de ALARCON, MADRID/ES, 5POZUELO DE ALARCON, MADRID/ ES Keywords: Genital / Reproductive system female, Pelvis, Oncology, MR, MR-Diffusion/Perfusion, PET-CT, Surgery, Radiation therapy / Oncology, Neoplasia DOI: 10.1594/ecr2012/C-1033 Any information contained in this pdf file is automatically generated from digital material submitted to EPOS by third parties in the form of scientific presentations. References to any names, marks, products, or services of third parties or hypertext links to third- party sites or information are provided solely as a convenience to you and do not in any way constitute or imply ECR's endorsement, sponsorship or recommendation of the third party, information, product or service. ECR is not responsible for the content of these pages and does not make any representations regarding the content or accuracy of material in this file. As per copyright regulations, any unauthorised use of the material or parts thereof as well as commercial reproduction or multiple distribution by any traditional or electronically based reproduction/publication method ist strictly prohibited. You agree to defend, indemnify, and hold ECR harmless from and against any and all claims, damages, costs, and expenses, including attorneys' fees, arising from or related to your use of these pages. -

Down Syndrome: a Serious Pediatric/ Podiatric Issue
Down Syndrome: A Serious Pediatric/ Podiatric Issue Early diagnosis and treatment can improve the lives of these patients. BY LOUIS J. DECARO, DPM The Responsibility and in.) From two years old until six cartilage, bone, and the support struc - of the Pediatric Podiatrist years old, the “normal foot” should ture of the skin. This creates signifi - The goal of any practitioner, no lose about one degree of the “ever - cant laxity from the feet up, thus be - matter what the specialty, should be sion” per year and at the age of six, ginning at a young age the lifelong to better the lives of their patients the heel should stand somewhat destruction of the kinetic chain. “Al - using every tool available without “straight up.” This allows an arch to most all of the conditions that affect bias. As podiatric physicians, we have be present. The foot support is on the the bones and joints of people with the unique ability to use all forms of outside, bringing the center of gravity Down syndrome arise from the abnor - medicine, including surgery, on our to a more neutral stance. Another phe - mal collagen found in Down syn - patients. A well-rounded podiatrist nomenon that is “supposed to hap - drome.” 1 The resulting effect in 88% should be someone who recognizes pen” as we grow older until around of the Down syndrome population is the implications of foot ailments at seven, is that our lower leg bones start hypotonia, with ligamentous laxity the earliest of ages in order to prevent adult problems. The feet are the foundation of the body, and from the first step a child From two years old until six years old, takes, deficiencies in the lower ex - the “normal foot” should lose about one degree tremity begin to create a destructive domino effect on the rest of the of the “eversion” per year and at the age of six, the heel human body. -

Current Management of Infantile Hemangiomas Sheilagh M
Current Management of Infantile Hemangiomas Sheilagh M. Maguiness, MD, and Ilona J. Frieden, MD During the past several years, there have been new advancements in the management of infantile hemangiomas (IHs). In many patients, no treatment is ever necessary—because IHs are well known for their natural history of spontaneous involution. However, a signif- icant minority of hemangiomas do require treatment. Moreover, they are very heteroge- neous, making the decision of when, how, and why to intervene quite variable. The least common but most important rationale for intervention is the presence of a life- or function- threatening complication, where prompt therapeutic intervention is a necessity. A much more common scenario is ulceration, where appropriate management is needed to expedite healing and control pain. Increasingly, the life-altering aspects of hemangioma are being recognized as a rationale for treatment because permanent scarring and disfigurement can result even if involution is complete. Treatments for IHs currently include topical, intrale- sional, and systemic therapies. Laser and surgical modalities are also sometimes used depending on the clinical scenario. In the absence of rigorous evidence-based studies, clinicians must carefully weigh the risks and benefits of medical or surgical treatments versus observation alone in tailoring management to the specific clinical situation at hand. Semin Cutan Med Surg 29:106-114 © 2010 Elsevier Inc. All rights reserved. nfantile hemangiomas (IHs) are the most common, benign ment uncertain, close follow-up and anticipatory guidance Ivascular tumors of infancy, present in 4% to 5% of the (so-called “active non-intervention”) may be the best ap- population.1 Hemangiomas have a characteristic clinical proach.3 appearance and predictable natural history. -

Diagnosis and Management of Infantile Hemangioma David H
CLINICAL REPORT Guidance for the Clinician in Rendering Pediatric Care Diagnosis and Management of Infantile Hemangioma David H. Darrow, MD, DDS, Arin K. Greene, MD, Anthony J. Mancini, MD, Amy J. Nopper, MD, the SECTION ON DERMATOLOGY, SECTION ON OTOLARYNGOLOGY–HEAD AND NECK SURGERY, and SECTION ON PLASTIC SURGERY abstract Infantile hemangiomas (IHs) are the most common tumors of childhood. Unlike other tumors, they have the unique ability to involute after proliferation, often leading primary care providers to assume they will resolve without intervention or consequence. Unfortunately, a subset of IHs rapidly develop complications, resulting in pain, functional impairment, or permanent disfigurement. As a result, the primary clinician has the task of determining which lesions require early consultation with a specialist. Although several recent reviews have been published, this clinical report is the first based on input from individuals representing the many specialties involved in the treatment of IH. Its purpose is to update the pediatric community regarding recent discoveries in IH pathogenesis, treatment, and clinical associations and This document is copyrighted and is property of the American to provide a basis for clinical decision-making in the management of IH. Academy of Pediatrics and its Board of Directors. All authors have filed conflict of interest statements with the American Academy of Pediatrics. Any conflicts have been resolved through a process approved by the Board of Directors. The American Academy of Pediatrics has neither solicited nor accepted any commercial involvement in the development of the content of this publication. NOMENCLATURE Clinical reports from the American Academy of Pediatrics benefit from The nomenclature and classification of vascular tumors and expertise and resources of liaisons and internal (American Academy malformations have evolved from clinical descriptions (“strawberry of Pediatrics) and external reviewers. -

Hemangioma Reticular Do Membro Inferior Associado a Anomalias
Localizador 11-014 Casos Clínicos DOI:10.4464/MC.2013.41.1.5052 Hemangioma reticular do membro inferior associado a anomalias estruturais congénitas: abordagem terapêutica Reticular hemangioma of the lower extremity associated with congenital structural anomalies: therapeutic approach P. Morais1,4, S. Magina1,4, A. Reis2,4, H. Pinto2, J. Amil Dias2, M. Rodrigues3, G. Silva3, F. Azevedo1, H. Guimarães3,4 1Department of Dermatovenereology. 2Department of Pediatrics. 3Department of Neonatology. Hospital de S. João. 4Faculty of Medicine. University of Porto. Portugal. Correspondencia: Paulo Morais e-mail: [email protected] Recibido: 22/2/2011 Aceptado: 27/12/2012 Resumo O hemangioma reticular é uma variante do hemangioma infantil descrita recentemente, que apresenta uma predilecção pela extremidade inferior e pelo períneo. Associa-se, frequentemente, a ulceração recalcitrante, anomalias ano-génito-urinárias e sacrais e, raramente, a sobrecarga cardíaca. Uma recém-nascida prematura apresentava uma mancha com aspecto reticular ao nível do membro inferior esquerdo, nádegas e períneo, dilatações venosas proeminentes na superfície lateral do membro envolvido, genitália externa dismórfica e ânus imperfurado. A ressonância magnética mostrou imperfuração anal, fístula rectovestibular, hipoplasia coccígea, cavidade siringomiélica medular e envolvimento hemangiomatoso retroperitoneal. As características clínicas e imagiológicas eram consistentes com o diagnóstico de hemangioma reticular com anomalias ventro-caudais. Os autores des- crevem a abordagem terapêutica adoptada nesta doente e a eficácia do encerramento assistido por vácuo de uma úlcera extensa e refractária aos cui- dados de penso convencionais. Palavras-chave: hemangioma reticular, hemangioma infantil, ulceração, encerramento assistido por vácuo, síndrome SACRAL, síndrome PELVIS, sín- drome LUMBAR. (P. Morais, S. Magina, A. Reis, H. Pinto, J. -

Diagnosis and Management of Infantile Hemangioma David H
CLINICAL REPORT Guidance for the Clinician in Rendering Pediatric Care Diagnosis and Management of Infantile Hemangioma David H. Darrow, MD, DDS, Arin K. Greene, MD, Anthony J. Mancini, MD, Amy J. Nopper, MD, the SECTION ON DERMATOLOGY, SECTION ON OTOLARYNGOLOGY–HEAD AND NECK SURGERY, and SECTION ON PLASTIC SURGERY abstract Infantile hemangiomas (IHs) are the most common tumors of childhood. Unlike other tumors, they have the unique ability to involute after proliferation, often leading primary care providers to assume they will resolve without intervention or consequence. Unfortunately, a subset of IHs rapidly develop complications, resulting in pain, functional impairment, or permanent disfigurement. As a result, the primary clinician has the task of determining which lesions require early consultation with a specialist. Although several recent reviews have been published, this clinical report is the first based on input from individuals representing the many specialties involved in the treatment of IH. Its purpose is to update the pediatric community regarding recent discoveries in IH pathogenesis, treatment, and clinical associations and This document is copyrighted and is property of the American to provide a basis for clinical decision-making in the management of IH. Academy of Pediatrics and its Board of Directors. All authors have filed conflict of interest statements with the American Academy of Pediatrics. Any conflicts have been resolved through a process approved by the Board of Directors. The American Academy of Pediatrics has neither solicited nor accepted any commercial involvement in the development of the content of this publication. NOMENCLATURE Clinical reports from the American Academy of Pediatrics benefit from The nomenclature and classification of vascular tumors and expertise and resources of liaisons and internal (American Academy malformations have evolved from clinical descriptions (“strawberry of Pediatrics) and external reviewers.