Diagnosis and Management of Infantile Hemangioma David H
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Use of Inflated Foley Catheters to Prevent Early Empty Pelvis Complications Following Pelvic Exenteration
ANTICANCER RESEARCH 35: 5543-5546 (2015) Use of Inflated Foley Catheters to Prevent Early Empty Pelvis Complications Following Pelvic Exenteration NICOLAE BACALBASA1, DANA TOMESCU2 and IRINA BALESCU3 1Carol Davila University of Medicine and Pharmacy, Bucharest, Romania; 2Fundeni Clinical Institute, Department of Anaethesia and Critical Care III, Bucharest, Romania; 3Ponderas Hospital, Bucharest, Romania Abstract. For most patients with bulky pelvic tumors, neoadjuvant chemo-irradiation is performed in order to pelvic exenteration remains the only curative option. diminish the local invasion and to transform the patient into Although initially reported as a palliative procedure, a candidate for a less extended resection. However, there nowadays it is rather performed with curative intent. Once are cases in which local invasion persists after neoadjuvant the resectional phase is ended, a large defect will remain at treatment and in which pelvic exenteration is needed. Since the level of the pelvic diaphragm, predisposing to severe Brunschwig reported it for the first time in 1948, this complications which are generically included under the surgical procedure has become the golden-standard for name of empty pelvis syndrome. It has been widely patients with locally invasive pelvic malignancies (3). demonstrated that this type of complication is associated Although the resectional phase has remained practically with severe mortality, even if the patient is free of any pelvic unchanged, the reconstructive phase has undergone multiple recurrence. We present the case of a 56-year-old patient improvements in order to improve the patient quality of submitted to total pelvic exenteration for locally invasive life. However, there are still cases in which a reconstruction previously chemo-irradiated cervical cancer who presented is not possible at the time of resection; in all such cases, a six months after surgery with a severe enteroperineal fistula. -
Detail Report
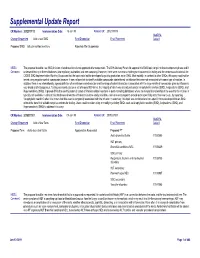
Supplemental Update Report CR Number: 2012319113 Implementation Date: 16-Jan-19 Related CR: 2012319113 MedDRA Change Requested Add a new SMQ Final Disposition Final Placement Code # Proposed SMQ Infusion related reactions Rejected After Suspension MSSO The proposal to add a new SMQ Infusion related reactions is not approved after suspension. The ICH Advisory Panel did approve this SMQ topic to go into the development phase and it Comment: underwent testing in three databases (two regulatory authorities and one company). However, there were numerous challenges encountered in testing and the consensus decision of the CIOMS SMQ Implementation Working Group was that the topic could not be developed to go into production as an SMQ. Most notably, in contrast to other SMQs, this query could not be tested using negative control compounds because it was not possible to identify suitable compounds administered via infusion that were not associated with some type of reaction. In addition, there is no internationally agreed definition of an infusion related reaction and the range of potential reactions associated with the large variety of compounds given by infusion is very broad and heterogenous. Testing was conducted on a set of around 500 terms, the majority of which was already included in Anaphylactic reaction (SMQ), Angioedema (SMQ), and Hypersensitivity (SMQ). It proved difficult to identify potential cases of infusion related reactions in post-marketing databases where the temporal relationship of the event to the infusion is typically not available. In clinical trial databases where this information is more easily available, users are encouraged to provide more specificity about the event, e.g., by reporting “Anaphylactic reaction” when it is known that this event is temporally associated with the infusion. -

Unusual Multiple Cutaneous Retiform Hemangioendothelioma on Forearm
Clinical and Diagnostic Pathology Research Article Unusual multiple cutaneous retiform hemangioendothelioma on forearm and neck misdiagnosed as angiosarcoma with metastasis Bin-cai Pan1, Chun-hua Wang1, Gui-fang Huang1, Xiao-ying Tian2 and Zhi Li3* 1Department of Pathology, Guangdong Tongjiang Hospital, Nanguo East Road, Shunde district, Foshan 528300, China 2School of Chinese Medicine, Hong Kong Baptist University 7, Baptist University Road, Kowloon Tong, Hong Kong, China 3Department of Pathology, The First Affiliated Hospital, Sun Yat-sen university.58, Zhongshan Road II,Guangzhou 510080, China Abstract Retiform hemangioendothelioma (RH) is extremely rare, and often involves the skin and subcutaneous tissues of distal extremities in young adults or children. Since its first description by Calonje in 1994, only a few primary multiple cases have been described in the literature. We present a case of unusual primary multiple RH on forearm and neck occurring in a 56 years old female patient. The patient presented with a slow-growing cutaneous plaque-like lesion on her left forearm, followed by another lesion at the site of neck for several years. In the skin biopsy examination, a diagnosis of angiosarcoma with cutaneous metastasis was made based on multiple lesions at different anatomic sites and vasoformative growth pattern with anastomosing channels under the microscopy. However, postoperative histological diagnosis of the lesion was primary multiple RH by thoroughly microscopical inspection and the presence of thin-walled interconnecting vascular channels arranged in a retiform pattern and absence of lymph node metastasis. Despite wide surgical excision with tumor-free margin, the tumor recurred at the neck 3 months after surgery. -

Infantile Hemangiomas
Infantile Hemangiomas Denise Metry, M.D. Q1. Hemangioma development is most closely associated with: A. Trauma during delivery B. In-utero hypoxia C. Geography D. Maternal smoking E. Maternal ingestion of strawberries Q1. Hemangioma development is most closely associated with: A. Trauma during delivery B. In-utero hypoxia C. Geography D. Maternal smoking E. Maternal ingestion of strawberries Q2. On average, the majority of hemangioma involution is complete by what age? A. 2 years B. 4 years C. 7 years D. 10 years E. They never involute Q2. On average, the majority of hemangioma involution is complete by what age? A. 2 years B. 4 years C. 7 years D. 10 years E. Never Hemangioma Growth/Involution • 80% of growth occurs in first 3-4 months • 80% of involution occurs by kindergarten Question 3 Q3. The best treatment option for this infant is… A. Nothing B. Topical timolol C. Oral propranolol D. Laser E. Surgery Q3. The best treatment option for this infant is… A. Nothing B. Topical timolol C. Oral propranolol D. Laser E. Surgery Timolol 0.5% gel-forming solution Best candidate: Superficial, relatively flat hemangioma Cosmetically sensitive location Infant < 3 months of age Apply 1 drop BID-TID up to 12 months of age Risk of toxicity appears low Monitoring not generally required Oral Propranolol • Non-selective beta-blocker • MOA? • Baseline maternal and infant history, heart rate • 20 mg/5 ml solution. Start at 0.5 mg/kg/day ÷ tid or bid and increase by 25% every 4 days to 2 mg/kg/day • Generally well-tolerated but hypoglycemia real risk • Continued until 12 to 18 months of age Question 4 Q4. -

Traumatic Arteriovenous Malformation of Cheek
AIJOC 10.5005/jp-journals-10003-1138 CASE REPORT Traumatic Arteriovenous Malformation of Cheek: A Case Report and Review of Literature Traumatic Arteriovenous Malformation of Cheek: A Case Report and Review of Literature Vadisha Srinivas Bhat, Rajeshwary Aroor, B Satheesh Kumar Bhandary, Shama Shetty ABSTRACT CASE REPORT Arteriovenous malformations (AVM) are congenital vascular A 31-year-old woman presented with swelling on right anomalies but are usually first noticed in childhood or adulthood. cheek of 6 months duration, which was insidious in onset Head and neck is the most common location for AVM. Extracranial lesions are rare compared to intracranial lesions. and gradually increasing in size. She had a history of The rapid enlargement of the malformation leading to symptoms undergoing dental treatment on the right upper premolar is usually triggered by trauma or hormonal changes of puberty tooth 1 month before the onset of swelling. There was no or pregnancy. Traumatic AVM of the head and neck are very history of any other trauma to the face. There were no rare. Here we report a case of AVM of cheek in an adult woman developed following a dental treatment. The diagnosis was symptoms of nasal disease. On examination, her general confirmed by imaging and was treated surgically after health state was good. There was a swelling measuring angiography and embolization. 3 × 2 cm on the right cheek near the nasolabial grove which Keywords: Arteriovenous malformation, Cheek, Dental was soft, compressible and nontender. The swelling was procedure, Angiography, Embolization. pulsatile (Figs 1 and 2). Nasal cavity and paranasal sinuses How to cite this article: Bhat VS, Aroor R, Bhandary BSK, were within normal limits. -

Orphanet Report Series Rare Diseases Collection
Marche des Maladies Rares – Alliance Maladies Rares Orphanet Report Series Rare Diseases collection DecemberOctober 2013 2009 List of rare diseases and synonyms Listed in alphabetical order www.orpha.net 20102206 Rare diseases listed in alphabetical order ORPHA ORPHA ORPHA Disease name Disease name Disease name Number Number Number 289157 1-alpha-hydroxylase deficiency 309127 3-hydroxyacyl-CoA dehydrogenase 228384 5q14.3 microdeletion syndrome deficiency 293948 1p21.3 microdeletion syndrome 314655 5q31.3 microdeletion syndrome 939 3-hydroxyisobutyric aciduria 1606 1p36 deletion syndrome 228415 5q35 microduplication syndrome 2616 3M syndrome 250989 1q21.1 microdeletion syndrome 96125 6p subtelomeric deletion syndrome 2616 3-M syndrome 250994 1q21.1 microduplication syndrome 251046 6p22 microdeletion syndrome 293843 3MC syndrome 250999 1q41q42 microdeletion syndrome 96125 6p25 microdeletion syndrome 6 3-methylcrotonylglycinuria 250999 1q41-q42 microdeletion syndrome 99135 6-phosphogluconate dehydrogenase 67046 3-methylglutaconic aciduria type 1 deficiency 238769 1q44 microdeletion syndrome 111 3-methylglutaconic aciduria type 2 13 6-pyruvoyl-tetrahydropterin synthase 976 2,8 dihydroxyadenine urolithiasis deficiency 67047 3-methylglutaconic aciduria type 3 869 2A syndrome 75857 6q terminal deletion 67048 3-methylglutaconic aciduria type 4 79154 2-aminoadipic 2-oxoadipic aciduria 171829 6q16 deletion syndrome 66634 3-methylglutaconic aciduria type 5 19 2-hydroxyglutaric acidemia 251056 6q25 microdeletion syndrome 352328 3-methylglutaconic -

Infantile Hemangioma: Factors Causing Recurrence After Propranolol Treatment
nature publishing group Basic Science Investigation | Articles Infantile hemangioma: factors causing recurrence after propranolol treatment Lei Chang1, Dongze Lv1, Zhang Yu1, Gang Ma1, Hanru Ying1, Yajing Qiu1, Yifei Gu1, Yunbo Jin1, Hui Chen1 and Xiaoxi Lin1 BACKGROUND: Propranolol is the first-choice treatment for hemangioma stem cells (hscs) with microRNAs (miRNAs) severe infantile hemangioma (IH). However, 10– 30% of that were differentially expressed between recurrent and non- lesions relapse after propranolol treatment. The mechanisms recurrent patients to preliminarily identify the factors underlying IH recurrence after propranolol treatment have not affecting IH recurrence after propranolol treatment. been completely elucidated. METHODS: This study combined an examination of hemo- METHODS dynamic changes with research regarding hemangioma stem Patients and Therapy cells (hscs) with differentially expressed microRNAs (miRNAs) The Declaration of Helsinki protocols were followed, and the to identify the factors affecting IH recurrence after propranolol procedures were approved by the ethics committee of Shanghai Ninth People’s Hospital (200806). The methods were conducted in treatment. Hemodynamic changes were monitored in 21 accordance with the relevant guidelines, and informed consent was recurrent cases using high-frequency color Doppler ultra- obtained from all subjects’ parents before samples of each subject’s sound, and hscs were treated with different concentrations of serum were collected. From December 2008 to May 2014, 1,015 IHs propranolol. The levels of differentially expressed miRNAs and were indicated for propranolol treatment after being assessed by two independent plastic surgeons, in accordance with the inclusion and the activity of related pathways were then compared between exclusion criteria (6). Propranolol was administered to patients at a 18 recurrent and 20 non-recurrent IH cases. -

Recognizing Common and Uncommon Birthmarks Harper N
Doctor should I be worried? Recognizing common and uncommon birthmarks Harper N. Price, MD, FAAD, FAAP Division Chief, Fellowship Director Friday, June 28, 10:50-11:35am Conflicts of interest: • None Learning objectives • Recognize common and less common congenital skin lesions in the outpatient setting • RED Vascular lesions: capillary malformations, hemangiomas, vascular tumors • BROWN Pigmented lesions: congenital nevi • BLUE dermal melanoyctosis • YELLOW/TAN Benign hamartomas: nevus sebaceous, connective tissue nevi • Developmental anomalies: aplasia cutis, hair collar sign • Identify those congenital skin lesions that require urgent referral and additional investigations Red birthmarks Classification of vascular anomalies • Incorrect nomenclature misunderstanding between colleagues and with patients • Incorrect nomenclature misdiagnosis • Accurate diagnosis is crucial for appropriate evaluation and management • Classification serves as a guide for clinicians Archaic terms • “Strawberry hemangioma” • “Cavernous hemangioma” • “Capillary hemangioma” • Historically speaking “hemangioma” has been used for vascular tumors and malformations Wassef M et al. Pediatr 2015 A simpler version Puttgen KB. Pediatr Clin N Am. 2014 Infantile hemangiomas (IH): classic vascular “tumor” • 4-10% of infants, head and neck • Most common soft tissue tumor of infancy • Present first few weeks of life • Proliferation of benign endothelial cells • Initial rapid growth followed by slow involution Infantile hemangiomas: risk factors • Low birth weight infants -

Vascular Anomalies Center Patient Information
VASCULAR ANOMALIES CENTER Patient Intake Form Phone: (832) 822-3800 Fax: (832) 825-9500 e-mail: [email protected] *** THIS FORM CAN BE COMPLETED BY A PARENT OR PHYSICIAN *** *** COMPLETED REFERRALS WILL BE REVIEWED WITHIN 5 BUSINESS DAYS *** ***IF SUBMITTED BY A PHYSICIAN, PLEASE INCLUDE THE CLINIC FACE SHEET WITH THE PATIENT’S DEMOGRAPHIC INFORMATION*** DATE OF REQUEST: ____________ COMPLETED BY: _________________ TCH MRN: ________________ PATIENT INFORMATION (PLEASE PRINT AND FILL OUT ALL BLANKS COMPLETELY) Last Name First Name & MI Age Date of Birth ☐M ☐F Home Address (Street) City, State and Zip Interpreter needed? ☐ Yes ☐ No If yes, what language? _______________ Parent/Guardian(s) Home Phone (check preferred) Work Phone Cell Phone ☐ ☐ ☐ Referring Physician Name (PCP and/or Subspecialist) Practice Contact Office Phone Office Fax CLINICAL INFORMATION (OVERVIEW OF VASCULAR ANOMALY) My child’s vascular anomaly is located:ALSKDJHFASKDHAKSDGASKHASKDFHASDKJHVASDKJFHASKDJFHSLDJHSDFH Left Right Both IF SKIN INVOLVEMENT OR DISCOLORATION Head and/or neck ☐ ☐ ☐ PRESENT, PLEASE SUBMIT A PHOTO. Upper extremity ☐ ☐ ☐ Lower extremity ☐ ☐ ☐ Trunk and/or abdomen ☐ ☐ ☐ My child’s vascular anomaly first appeared? Since then, the vascular anomaly has: ☐ on a prenatal exam ☐ become smaller ☐ at birth ☐ stayed the same ☐ within 1 month of birth ☐ become larger ☐ within 1 year of birth ☐ at ______ years of age Does your child have a specific diagnosis? (Check all that have been told to you.) ☐ Hemangioma ☐ Port wine stain (capillary -

Multipotential Stem Cells Recapitulate Human Infantile Hemangioma in Immunodeficient Mice
Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice Zia A. Khan, … , John B. Mulliken, Joyce Bischoff J Clin Invest. 2008;118(7):2592-2599. https://doi.org/10.1172/JCI33493. Research Article Angiogenesis Infantile hemangioma is a benign endothelial tumor composed of disorganized blood vessels. It exhibits a unique life cycle of rapid postnatal growth followed by slow regression to a fibrofatty residuum. Here, we have reported the isolation of multipotential stem cells from hemangioma tissue that give rise to hemangioma-like lesions in immunodeficient mice. Cells were isolated based on expression of the stem cell marker CD133 and expanded from single cells as clonal populations. The CD133-selected cells generated human blood vessels 7 days after implantation in immunodeficient mice. Cell retrieval experiments showed the cells could again form vessels when transplanted into secondary recipients. The human vessels expressed GLUT-1 and merosin, immunodiagnostic markers for infantile hemangioma. Two months after implantation, the number of blood vessels diminished and human adipocytes became evident. Lentiviral expression of GFP was used to confirm that the hemangioma-derived cells formed the blood vessels and adipocytes in the immunodeficient mice. Thus, when transplanted into immunodeficient mice, hemangioma-derived cells recapitulated the unique evolution of infantile hemangioma — the formation of blood vessels followed by involution to fatty tissue. In summary, this study identifies a stem cell as the cellular origin of infantile hemangioma and describes for what we believe is the first time an animal model for this common tumor of infancy. Find the latest version: https://jci.me/33493/pdf Research article Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice Zia A. -

What Is Infantile Hemangioma?
What Is Infantile Hemangioma? Infantile hemangiomas (he-man-jee-O-muhs), sometimes called “strawberry marks,” are benign tumors formed from the overgrowth of blood vessels on or under the skin. Symptoms A hemangioma may be present at birth, but more often appears during the first several months of life. It starts out as a flat red mark anywhere on the body, most often on the face, scalp, chest or back. Usually a child has only one mark. Some children may have more than one, particularly if they're part of a multiple birth. During your child's first year, the red mark grows rapidly (proliferation) and becomes a spongy mass that protrudes from the skin. The hemangioma then enters a rest phase and then eventually slowly starts to improve over a span of up to 9 years. While most infantile hemangiomas go away on their own in time, others can lead to permanent scarring if left untreated: • 1 out of 3 facial infantile hemangiomas will result in disfigurement from permanent soft tissue distortion, which can be truly life-altering. • 69% of infantile hemangiomas can leave permanent residual lesions (i.e., scares, extra skin or extra fatty tissue). Diagnosis A hemangioma is diagnosed based on appearance. Diagnostic tests aren’t usually needed. Causes The causes of infantile hemangioma are not clear. However, we know certain babies are at higher risk of infantile hemangioma. Risk factors include: • Babies with lower birth weight • Females • Caucasians • Premature babies • Multiple Gestation It is important to know that you did nothing wrong and it is not your fault that your baby has infantile hemangioma. -

What Is Your Diagnosis?
Photo Quiz What Is Your Diagnosis? CUTIS Do Not Copy A 6-week-old male infant was referred to the dermatology department for evaluation of enlarging facial lesions noted shortly after birth. The patient was delivered at 36 weeks’ gestation by normal spontaneous vaginal delivery with no perinatal complications. His growth and development were otherwise nor- mal. Physical examination revealed large, bright red, nonconfluent macules and plaques in a bilateral temporal distribution extending medially to both eye- lids and laterally to the scalp. PLEASE TURN TO PAGE 119 FOR DISCUSSION Shilpa S. Sawardekar, MD; Heather L. Salvaggio, MD; Andrea L. Zaenglein, MD Drs. Sawardekar and Salvaggio were from and Dr. Zaenglein is from the Department of Dermatology and Pediatrics, Penn State Milton S. Hershey Medical Center, Hershey, Pennsylvania. Dr. Sawardekar currently is from the Department of Dermatology, Eastern Virginia Medical School, Norfolk. Dr. Salvaggio currently is from the Division of Dermatology, Bassett Medical Center, Cooperstown, New York. The authors report no conflict of interest. Correspondence: Andrea L. Zaenglein, MD, Department of Dermatology, HU14, Penn State Milton S. Hershey Medical Center, 500 University Dr, Hershey, PA 17033 ([email protected]). WWW.CUTIS.COM VOLUME 92, SEPTEMBER 2013 113 Copyright Cutis 2013. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher. Photo Quiz Discussion The Diagnosis: PHACE Syndrome CUTIS HACE (posterior fossa brain malformations, syndrome is made in the following scenarios: a large hemangiomas, arterial anomalies, cardiac hemangioma greater than 5 cm plus 1 minor crite- Pdefects and coarctation of the aorta, eye and ria, a hemangioma of the neck or upper torso plus endocrine abnormalities) syndrome is a neurocu- 1 major or 2 minor criteria, or no hemangioma plus taneousDo disorder characterized Not by a spectrum of 2 major Copy criteria.4 abnormalities.