Pediatric Vascular Disorders Chelsea R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Hepatic Outflow Obstruction (Budd-Chiari Syndrome) Case Due
OLGU SUNUMU / CASE REPORT Gülhane Tıp Derg 2014;56: 110-113 © Gülhane Askeri Tıp Akademesi 2014 doi: 10.5455/gulhane.13835 A Hepatic Outflow Obstruction (Budd-Chiari Syndrome) Case Due to Multiple Hypercoagulable Status Yusuf Yazgan (*), Kemal Oncu (*), Mustafa Kaplan (*), Alpaslan Tanoglu (*), İrfan Küçük (*), Halil Onur Ozari (*), Levent Demirturk (*), Murat Velioglu (**) Introduction: SUMMARY Primary Budd-Chiari syndrome (BCS) is a rare disorder We present here a case of a 22-year-old male patient with Budd- caused by thrombosis of the hepatic veins or the terminal Chiari syndrome owing to alliance of multiple hypercoagulable portion of the inferior vena cava. Its estimated incidence ranges conditions. The patient was admitted to our hospital for from 0.2 to 0.8 per million per year (1). In developed countries, assesment of hepatosplenomegaly and ascites. By doppler the hepatic vein thrombosis is the most frequent presentation, ultrasonography, computed tomography and vena cavagraphy, however in developing countries membranous blockade is the Budd-Chiari syndrome was diagnosed. Results of diagnostic tests most common cause of the BCS. BCS is closely associated exhibited decreased activity, decreased antigenic concentration of Antithrombin, low protein C activity, heterozygote Factor with prothrombotic conditions especially with hematologic V Leiden mutation. In clinical progress, acute severe hepatic disorders. For example primary myeloproliferative diseases failure with encephalopathy occured and the patient was (i.e. polycythemia vera) may account for nearly half of the transferred to an another medical center for liver transplantation. all cases (2). Tumors, pregnancy, infections are also other common reasons. Oral contraceptives raise the risk of BCS Key words: Budd-Chiari Syndrome, Antithrombin deficiency, low by nearly two-fold (3). -

Uniform Faint Reticulate Pigment Network - a Dermoscopic Hallmark of Nevus Depigmentosus
Our Dermatology Online Letter to the Editor UUniformniform ffaintaint rreticulateeticulate ppigmentigment nnetworketwork - A ddermoscopicermoscopic hhallmarkallmark ooff nnevusevus ddepigmentosusepigmentosus Surit Malakar1, Samipa Samir Mukherjee2,3, Subrata Malakar3 11st Year Post graduate, Department of Dermatology, SUM Hospital Bhubaneshwar, India, 2Department of Dermatology, Cloud nine Hospital, Bangalore, India, 3Department of Dermatology, Rita Skin Foundation, Kolkata, India Corresponding author: Dr. Samipa Samir Mukherjee, E-mail: [email protected] Sir, ND is a form of cutaneous mosaicism with functionally defective melanocytes and abnormal melanosomes. Nevus depigmentosus (ND) is a localized Histopathologic examination shows normal to hypopigmentation which most of the time is congenital decreased number of melanocytes with S-100 stain and and not uncommonly a diagnostic challenge. ND lesions less reactivity with 3,4-dihydroxyphenylalanine reaction are sometimes difficult to differentiate from other and no melanin incontinence [2]. Electron microscopic hypopigmented lesions like vitiligo, ash leaf macules and findings show stubby dendrites of melanocytes nevus anemicus. Among these naevus depigmentosus containing autophagosomes with aggregates of poses maximum difficulty in differentiating from ash melanosomes. leaf macules because of clinical as well as histological similarities [1]. Although the evolution of newer diagnostic For ease of understanding the pigmentary network techniques like dermoscopy has obviated the -

Use of Inflated Foley Catheters to Prevent Early Empty Pelvis Complications Following Pelvic Exenteration
ANTICANCER RESEARCH 35: 5543-5546 (2015) Use of Inflated Foley Catheters to Prevent Early Empty Pelvis Complications Following Pelvic Exenteration NICOLAE BACALBASA1, DANA TOMESCU2 and IRINA BALESCU3 1Carol Davila University of Medicine and Pharmacy, Bucharest, Romania; 2Fundeni Clinical Institute, Department of Anaethesia and Critical Care III, Bucharest, Romania; 3Ponderas Hospital, Bucharest, Romania Abstract. For most patients with bulky pelvic tumors, neoadjuvant chemo-irradiation is performed in order to pelvic exenteration remains the only curative option. diminish the local invasion and to transform the patient into Although initially reported as a palliative procedure, a candidate for a less extended resection. However, there nowadays it is rather performed with curative intent. Once are cases in which local invasion persists after neoadjuvant the resectional phase is ended, a large defect will remain at treatment and in which pelvic exenteration is needed. Since the level of the pelvic diaphragm, predisposing to severe Brunschwig reported it for the first time in 1948, this complications which are generically included under the surgical procedure has become the golden-standard for name of empty pelvis syndrome. It has been widely patients with locally invasive pelvic malignancies (3). demonstrated that this type of complication is associated Although the resectional phase has remained practically with severe mortality, even if the patient is free of any pelvic unchanged, the reconstructive phase has undergone multiple recurrence. We present the case of a 56-year-old patient improvements in order to improve the patient quality of submitted to total pelvic exenteration for locally invasive life. However, there are still cases in which a reconstruction previously chemo-irradiated cervical cancer who presented is not possible at the time of resection; in all such cases, a six months after surgery with a severe enteroperineal fistula. -

Recurrent Targetoid Hemosiderotic Hemangioma in a 26-Year-Old Man
CASE REPORT Recurrent Targetoid Hemosiderotic Hemangioma in a 26-Year-Old Man LT Sarah Broski Gendernalik, DO, MC (FS), USN LT James D. Gendernalik, DO, MC (FS), USN A 26-year-old previously healthy man presented with a 6-mm violaceous papule that had a surrounding 1.5-cm annular, nonblanching, erythematous halo on the right-sided flank. The man reported the lesion had been recurring for 4 to 5 years, flaring every 4 to 5 months and then slowly disap - pearing until the cycle recurred. Targetoid hemosiderotic hemangioma was clinically diagnosed. The lesion was removed by means of elliptical excision and the condition resolved. The authors discuss the clinical appearance, his - tology, and etiology of targetoid hemosiderotic heman - giomas. J Am Osteopath Assoc . 2011;111(2);117-118 argetoid hemosiderotic hemangiomas (THHs) are a com - Tmonly misdiagnosed presentation encountered in the primary care setting. In the present case report, we aim to pro - Figure. A 6-mm violaceous papule with a surrounding 1.5-cm annular, nonblanching, erythematous halo in a 26-year-old man. vide general practitioners with an understanding of the clin - ical appearance, pathology, and prognosis of THH. Report of Case A 26-year-old previously healthy man presented to our primary around it and itched and burned each time it developed. The care clinic with a 6-mm violaceous papule with a surrounding lesion faded completely to normal-appearing skin between 1.5-cm annular, nonblanching, erythematous halo on the right- episodes, without evidence of a papule or postinflammatory sided flank ( Figure ). The patient stated that the lesion had hyperpigmentation. -

Hemangiosarcoma Philip J
Ettinger & Feldman – Textbook of Veterinary Internal Medicine Client Information Sheet Hemangiosarcoma Philip J. Bergman What is hemangiosarcoma? Hemangiosarcoma (HSA; angiosarcoma or malignant hemangioendothelioma) is an extremely aggressive tumor of blood vessel origin. Because blood vessels are present throughout the body, virtually any site in the body can have HSA. HSA occurs most frequently in dogs (approximately 2% of all tumors) and the most common site is the spleen. However, additional common sites include the heart, liver, muscle, lung skin, bones, kidney, brain, abdomen, and oral cavity. In three large canine splenic disease studies encompassing approximately 2000 dogs, a “rule of two thirds” was found suggesting that approximately two thirds of dogs with a splenic mass have a cancer (therefore one third are not malignant) and two thirds of the malignant tumors of the spleen are HSA. HSA is a disease generally of older dogs and cats with an average onset of 9 to 10 years; however, there are reports of extremely young dogs and cats with this disease (5 to 6 months to a few years of age). German shepherd dogs are most commonly diagnosed with HSA; however, other large breed dogs such as golden retrievers and Labrador retrievers may also be overrepresented. In cats, the most common breed is the domestic shorthair. The cause of HSA in dogs and cats is presently unknown. Exposures to toxins such as chemicals, insecticides, and radiation have been reported in humans to be associated with HSA. Ultraviolet light exposure from the sun may be a potential cause of HSA in dogs, as HSAs of the skin are commonly seen in dogs with light hair and poor pigmentation (e.g., Salukis, Whippets, and white Bulldogs). -
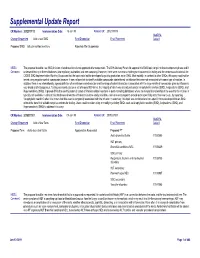
Detail Report
Supplemental Update Report CR Number: 2012319113 Implementation Date: 16-Jan-19 Related CR: 2012319113 MedDRA Change Requested Add a new SMQ Final Disposition Final Placement Code # Proposed SMQ Infusion related reactions Rejected After Suspension MSSO The proposal to add a new SMQ Infusion related reactions is not approved after suspension. The ICH Advisory Panel did approve this SMQ topic to go into the development phase and it Comment: underwent testing in three databases (two regulatory authorities and one company). However, there were numerous challenges encountered in testing and the consensus decision of the CIOMS SMQ Implementation Working Group was that the topic could not be developed to go into production as an SMQ. Most notably, in contrast to other SMQs, this query could not be tested using negative control compounds because it was not possible to identify suitable compounds administered via infusion that were not associated with some type of reaction. In addition, there is no internationally agreed definition of an infusion related reaction and the range of potential reactions associated with the large variety of compounds given by infusion is very broad and heterogenous. Testing was conducted on a set of around 500 terms, the majority of which was already included in Anaphylactic reaction (SMQ), Angioedema (SMQ), and Hypersensitivity (SMQ). It proved difficult to identify potential cases of infusion related reactions in post-marketing databases where the temporal relationship of the event to the infusion is typically not available. In clinical trial databases where this information is more easily available, users are encouraged to provide more specificity about the event, e.g., by reporting “Anaphylactic reaction” when it is known that this event is temporally associated with the infusion. -

Angiokeratoma of the Scrotum (Fordyce Type) Associated with Angiokeratoma of the Oral Cavity
208 Letters to the Editor anti-thyroperoxidas e antibody in addition to, or, less Yamada A. Antineutrophil cytoplasmic autoantibody- likely, instead of MPO-ANCA cannot be excluded. positive crescentric glomerulonephritis associated with thi- amazole therapy. Nephron 1996; 74: 734–735. Vesiculo-bullous SLE has been reported to respond 6. Cooper D. Antithyroid drugs. N Engl J Med 1984; 311: to dapsone (15). However, in our patient, an early 1353–1362. aggressive treatment with steroid pulse therapy and 7. Yung RL, Richardson BC. Drug-induced lupus. Rheum plasmapheresis was mandatory because of her life- Dis Clin North Am 1994; 20: 61–86. threatening clinical condition. The contributory factors, 8. Hess E. Drug-related lupus. N Engl J Med 1988; 318: 1460–1462. such as an environmental trigger or an immunological 9. Sato-Matsumura KC, Koizumi H, Matsumura T, factor, for the presence of a serious illness in this patient Takahashi T, Adachi K, Ohkawara A. Lupus eryth- remain to be elucidated. The mechanism by which ematosus-like syndrome induced by thiamazole and methimazole induces SLE-like reactions is unclear. propylthiouracil. J Dermatol 1994; 21: 501–507. 10. Wing SS, Fantus IG. Adverse immunologic eVects of antithyroid drugs. Can Med Assoc J 1987; 136: 121–127. 11. Condon C, Phelan M, Lyons JF. Penicillamine-induced REFERENCES type II bullous systemic lupus erythematosus. Br J Dermatol 1997; 136: 474–475. 1. Alarcon-Segovia D. Drug induced lupus syndromes. Mayo 12. Stankus S, Johnson N. Propylthiouracil-induced hyper- Clin Proc 1969; 44: 664–681.2. sensitivity vasculitis presenting as respiratory failure. Chest 2. Cush JJ, Goldings EA. -

1-Dr Zamirian
IJMS Vol 32, No 1, March 2007 Original Article Contrast Enhanced Echocardiography for Detection of Intrapulmonary Shunts in Liver Transplant Candidates M. Zamirian, A. Aslani Abstract Background: Intrapulmonary vascular abnormalities associated with liver cirrhosis may result in intrapulmonary right-to-left shunt and hypoxemia. The aim of this study was to use con- trast enhanced echocardiography to detect intrapulmonary vascular abnormalities in patients with liver cirrhosis candi- dates for liver transplantation. Methods: One hundred and two adult patients underwent contrast enhanced echocardiography to determine the prevalence of in- trapulmonary right-to-left shunt and its relationship to the severity of hepatic disease, arterial oxygenation, and spider angioma. Results: The rate of patients with positive and negative con- trast enhanced echocardiography was 44% and 56%, respec- tively. There was no significant difference in age, sex, or eti- ology of liver cirrhosis in patients with and without intrapul- monary shunt. Patients with intrapulmonary right-to-left shunt had more severe hepatic disease compared with those without shunt (Child-Pugh score 12±2 vs 8±2). There was significant difference in the partial arterial oxygen pressure (PaO2) values + + in patients with grade 3 to 4 left ventricular opacification by microbubbles compared with those without evidence of in- trapulmonary right-to-left shunt (64±6 vs 82±10 mmHg). Twenty eight of the patients with intrapulmonary right-to-left shunt had cutaneous spider angioma. Conclusion: The findings suggest that there was a significant relation between severity of liver cirrhosis and presence of intrapulmonary right-to-left shunt or severity of hypoxemia. The data also indicate that cirrhotic patients with cutaneous spider angioma most likely have the shunt. -

Angiokeratoma of the Scrotum (Fordyce)
Keio Journal of Medicine Vol. 1, No. 1, January, 1952 ANGIOKERATOMA OF THE SCROTUM (FORDYCE) MASAKATSU IZAKI Department of Dermatology, School of Medicine, Keio University Since Fordyce, in 1896, first described a case of angiokeratoma of the scrotum, many authors have reported and discussed about this dermatosis. However the classification and the nomenclature of this skin disease still remain in a state of confusion. Recently I had a chance to see the report of Robinson and Tasker (1946)(14), discussing the nomenclature of this condition, which held my attention considerably. In this paper I wish to report statistical observation concerning the incidences of this dermatosis among Japanese males, and histopathological studies made in 5 cases of this condition. STATISTICALOBSERVATION It must be first pointed out that this study was made along with the statistical study on angioma senile and same persons were examined in both dermatosis (ref. Studies on Senile Changes in the Skin I. Statistical Observation; Journal of the Keio Medical Society Vol. 28, No. 2, p. 59, 1951). The statistics was handled by the small sampling method. Totals of persons examined were 1552 males. Their ages varied from 16 to 84 years, divided into seven groups: i.e. the late teen-agers (16-20), persons of the third decade (21-30), of the fourth decade (31-40), of the fifth decade (41-50), of the sixth decade (51-60), of the seventh decade (61-70) and a group of persons over 71 years of age. The number of persons and the incidence of this condition in each group are summarized briefly in Table 1. -

Kaposiform Hemangioendothelioma with Kasabach-Merritt Syndrome Mistaken for Child Abuse in a Newborn
Kaposiform Hemangioendothelioma With Kasabach-Merritt Syndrome Mistaken for Child Abuse in a Newborn Amanda A. Cyrulnik, MD; Manju C. Dawkins, MD; Gert J. Smalberger, MD; Scott Young, MD; Ranon E. Mann, MD; Mark I. Jacobson, MD; Adam J. Friedman, MD Practice Points Vascular tumors in dermatology may mimic child abuse. Even when all signs favor abuse, a nonresolving lesion should alarm clinicians to consider an alter- nate diagnosis. Kasabach-Merritt syndrome is a serious and potentially life-threatening condition that requires prompt evaluation. Kaposiform hemangioendotheliomaCUTIS is a rare vas- elevated D-dimer levels, confirming a diagnosis of cular neoplasm of childhood that may have an Kasabach-Merritt syndrome (KMS). alarming and potentially misleading clinical pre- Cutis. 2014;93:E17-E20. sentation. Awareness of this entity is important to provide appropriate and immediate medical care. Case Report We report the case of a 24-day-old female new- A 24-day-old female newborn presented to a hospi- born who presented with a large bruiselike lesion tal with a large bruiselike lesion on the left leg. A on theDo left leg. A diagnosis ofNot cellulitis suspected diagnosis Copy of cellulitis suspected to be secondary to to be secondary to child abuse was made and the child abuse was made and the patient subsequently patient subsequently was placed in foster care; was placed in foster care; however, the lesion did not however, the lesion did not resolve after treatment resolve after treatment and relocation. At 69 days of and relocation. On reevaluation at our institution, age, the patient was readmitted, now to our hospi- physical examination revealed a round, 34-cm, tal, after the lesion persisted and had progressively violaceous, indurated, fixed, nonblanching, non- expanded. -

Pityriasis Alba Revisited: Perspectives on an Enigmatic Disorder of Childhood
Pediatric ddermatologyermatology Series Editor: Camila K. Janniger, MD Pityriasis Alba Revisited: Perspectives on an Enigmatic Disorder of Childhood Yuri T. Jadotte, MD; Camila K. Janniger, MD Pityriasis alba (PA) is a localized hypopigmented 80 years ago.2 Mainly seen in the pediatric popula- disorder of childhood with many existing clinical tion, it primarily affects the head and neck region, variants. It is more often detected in individuals with the face being the most commonly involved with a darker complexion but may occur in indi- site.1-3 Pityriasis alba is present in individuals with viduals of all skin types. Atopy, xerosis, and min- all skin types, though it is more noticeable in those with eral deficiencies are potential risk factors. Sun a darker complexion.1,3 This condition also is known exposure exacerbates the contrast between nor- as furfuraceous impetigo, erythema streptogenes, mal and lesional skin, making lesions more visible and pityriasis streptogenes.1 The term pityriasis alba and patients more likely to seek medical atten- remains accurate and appropriate given the etiologic tion. Poor cutaneous hydration appears to be a elusiveness of the disorder. common theme for most riskCUTIS factors and may help elucidate the pathogenesis of this disorder. The Epidemiology end result of this mechanism is inappropriate mel- Pityriasis alba primarily affects preadolescent children anosis manifesting as hypopigmentation. It must aged 3 to 16 years,4 with onset typically occurring be differentiated from other disorders of hypopig- between 6 and 12 years of age.5 Most patients are mentation, such as pityriasis versicolor alba, vitiligo, younger than 15 years,3 with up to 90% aged 6 to nevus depigmentosus, and nevus anemicus. -

Genes in Eyecare Geneseyedoc 3 W.M
Genes in Eyecare geneseyedoc 3 W.M. Lyle and T.D. Williams 15 Mar 04 This information has been gathered from several sources; however, the principal source is V. A. McKusick’s Mendelian Inheritance in Man on CD-ROM. Baltimore, Johns Hopkins University Press, 1998. Other sources include McKusick’s, Mendelian Inheritance in Man. Catalogs of Human Genes and Genetic Disorders. Baltimore. Johns Hopkins University Press 1998 (12th edition). http://www.ncbi.nlm.nih.gov/Omim See also S.P.Daiger, L.S. Sullivan, and B.J.F. Rossiter Ret Net http://www.sph.uth.tmc.edu/Retnet disease.htm/. Also E.I. Traboulsi’s, Genetic Diseases of the Eye, New York, Oxford University Press, 1998. And Genetics in Primary Eyecare and Clinical Medicine by M.R. Seashore and R.S.Wappner, Appleton and Lange 1996. M. Ridley’s book Genome published in 2000 by Perennial provides additional information. Ridley estimates that we have 60,000 to 80,000 genes. See also R.M. Henig’s book The Monk in the Garden: The Lost and Found Genius of Gregor Mendel, published by Houghton Mifflin in 2001 which tells about the Father of Genetics. The 3rd edition of F. H. Roy’s book Ocular Syndromes and Systemic Diseases published by Lippincott Williams & Wilkins in 2002 facilitates differential diagnosis. Additional information is provided in D. Pavan-Langston’s Manual of Ocular Diagnosis and Therapy (5th edition) published by Lippincott Williams & Wilkins in 2002. M.A. Foote wrote Basic Human Genetics for Medical Writers in the AMWA Journal 2002;17:7-17. A compilation such as this might suggest that one gene = one disease.