CHICKPEA BREEDING and MANAGEMENT This Page Intentionally Left Blank CHICKPEA BREEDING and MANAGEMENT
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Social Mobility: the Meaning, Types and Factors Responsible for Social Mobilit
Social Mobility: The Meaning, Types and Factors Responsible for Social Mobilit Dr. Kumar Satyendra Yadav, Assistant Professor, Statistics Dept.. Patna University, Patna Meaning of Social Mobility: Mobility stands for shift, change and movement. The change may be of a place or from one position to another. Further, change is value free i.e it cannot be said that change is for good or bad. When we prefix „social‟ along with mobility it would imply that people or individual occupying a social position, move to another position or status. In the social ladder this movement may be upward or downward or it may be inter-generational or intra- generational. In short, social mobility stands for change in the position of an individual or a group of individuals from one status to another. On mobility Sorokin was the first sociologist who wrote a book “Social and Cultural Mobility”. He was of the opinion that there is no society which is closed (Caste System in India) and no society which is completely open (Class System). He further contended that no two societies are exactly same in the amount of movement allowed or discouraged. Further the speed of movement or change may differ from one period of time to another. The rate of change depends upon the level of modernization of a given society. In this sense, mobility “provides the individual with more or less of the benefits which his economy and society have to offer.” A rickshaw puller‟s son becomes a lawyer; a clerk‟s son becomes a doctor. In each case, a change in role between father and son provides the latter with more of the good things of life. -

Diverse Genetic Origin of Indian Muslims: Evidence from Autosomal STR Loci
Journal of Human Genetics (2009) 54, 340–348 & 2009 The Japan Society of Human Genetics All rights reserved 1434-5161/09 $32.00 www.nature.com/jhg ORIGINAL ARTICLE Diverse genetic origin of Indian Muslims: evidence from autosomal STR loci Muthukrishnan Eaaswarkhanth1,2, Bhawna Dubey1, Poorlin Ramakodi Meganathan1, Zeinab Ravesh2, Faizan Ahmed Khan3, Lalji Singh2, Kumarasamy Thangaraj2 and Ikramul Haque1 The origin and relationships of Indian Muslims is still dubious and are not yet genetically well studied. In the light of historically attested movements into Indian subcontinent during the demic expansion of Islam, the present study aims to substantiate whether it had been accompanied by any gene flow or only a cultural transformation phenomenon. An array of 13 autosomal STR markers that are common in the worldwide data sets was used to explore the genetic diversity of Indian Muslims. The austere endogamy being practiced for several generations was confirmed by the genetic demarcation of each of the six Indian Muslim communities in the phylogenetic assessments for the markers examined. The analyses were further refined by comparison with geographically closest neighboring Hindu religious groups (including several caste and tribal populations) and the populations from Middle East, East Asia and Europe. We found that some of the Muslim populations displayed high level of regional genetic affinity rather than religious affinity. Interestingly, in Dawoodi Bohras (TN and GUJ) and Iranian Shia significant genetic contribution from West Asia, especially Iran (49, 47 and 46%, respectively) was observed. This divulges the existence of Middle Eastern genetic signatures in some of the contemporary Indian Muslim populations. -

Y-Chromosomal and Mitochondrial SNP Haplogroup Distribution In
Open Access Austin Journal of Forensic Science and Criminology Review Article Y-Chromosomal and Mitochondrial SNP Haplogroup Distribution in Indian Populations and its Significance in Disaster Victim Identification (DVI) - A Review Based Molecular Approach Sinha M1*, Rao IA1 and Mitra M2 1Department of Forensic Science, Guru Ghasidas Abstract University, India Disaster Victim Identification is an important aspect in mass disaster cases. 2School of Studies in Anthropology, Pt. Ravishankar In India, the scenario of disaster victim identification is very challenging unlike Shukla University, India any other developing countries due to lack of any organized government firm who *Corresponding author: Sinha M, Department of can make these challenging aspects an easier way to deal with. The objective Forensic Science, Guru Ghasidas University, India of this article is to bring spotlight on the potential and utility of uniparental DNA haplogroup databases in Disaster Victim Identification. Therefore, in this article Received: December 08, 2016; Accepted: January 19, we reviewed and presented the molecular studies on mitochondrial and Y- 2017; Published: January 24, 2017 chromosomal DNA haplogroup distribution in various ethnic populations from all over India that can be useful in framing a uniparental DNA haplogroup database on Indian population for Disaster Victim Identification (DVI). Keywords: Disaster Victim identification; Uniparental DNA; Haplogroup database; India Introduction with the necessity mentioned above which can reveal the fact that the human genome variation is not uniform. This inconsequential Disaster Victim Identification (DVI) is the recognized practice assertion put forward characteristics of a number of markers ranging whereby numerous individuals who have died as a result of a particular from its distribution in the genome, their power of discrimination event have their identity established through the use of scientifically and population restriction, to the sturdiness nature of markers to established procedures and methods [1]. -

Hari Nair 915-747-7544 | [email protected] | Web |
Hari Nair 915-747-7544 j [email protected] j web j Appointments The University of Texas at El Paso El Paso, TX Assistant Professor, Department of Physics Sep. 2018 { present The University of Texas at El Paso El Paso, TX Visiting Assistant Professor, Department of Physics Sep. 2017 { Sep. 2018 Colorado State University Fort Collins, CO, USA Postdoctoral Researcher, Department of Physics Feb 2016 { Aug 2017 University of Johannesburg Johannesburg, South Africa Postdoctoral Researcher, Department of Physics Oct 2014 { Dec 2015 JCNS-2 & Peter Grunberg Institute J¨ulich, Germany Scientific Staff, Forschungszentrum J¨ulichGmbH Apr 2011 { Jul2014 Indian Institute of Science Bangalore, India Postdoctoral Researcher, Department of Physics Feb 2011 { Apr 2011 Indian Institute of Science Bangalore, India Researcher, Department of Physics Oct 2009 { Sep 2010 Visiting positions Universitat zu K¨oln K¨oln,Germany Researcher, II. Physikalisches Institut Oct 2010 { Jan 2011 Forschungszentrum J¨ulich GmbH J¨ulich, Germany Visiting Researcher, Institut fur Festk¨orperforschung (IFF) Jun 2009 { Sep 2009 Max Planck Institute for Chemical Physics of Solids Dresden, Germany Visiting Researcher May 2008 { Jul 2008 Leibniz Institute for Solid State and Materials Research Dresden, Germany Visiting Researcher Oct 2006 { Dec 2006 Education Indian Institute of Science Bangalore, India PhD in experimental condensed matter physics Jun. 2002 { Dec. 2009 Mahatma Gandhi University Kerala, India MSc in Physics from C. M. S. College 2000 { 2002 Kerala University Kerala, India BSc in Physics from T. K. M. College 1997 { 2000 Grants/ Awards/ Honours 2020: (1) coPI in National Science Foundation Major Research Instrumentation program award to UTEP to acquire an MPMS. -

Dr. Jhillu Singh Yadav – Scientist Par Excellence a Tribute
Issue in Honor of Dr. Jhillu S. Yadav ARKIVOC 2016 (ii) 1-8 Dr. Jhillu Singh Yadav – Scientist par excellence A Tribute Dedicated to Dr. Jhillu Singh Yadav on the occasion of his outstanding contribution to organic synthetic chemistry I feel privileged to pay tribute to Dr. J. S. Yadav, who has been magnificent as a co-mentor (during my initial career as research fellow) and colleague after I joined the Institute as a scientist. Dr. Yadav was born on the 4th of August, 1950 in Azamgarh, Uttar Pradesh, India. After school in Uttar Pradesh (largest state in India), he Joined Banaras Hindu University, Varanasi (one of the oldest Universities in India) in 1968 and pursued his Bachelors and Masters degrees. He completed his Ph.D in a span of three years from the National Chemical Laboratories, Pune and was awarded a doctorate from Maharaja Sayajirao University of Baroda in 1976 where he was involved in Isolongifolene chemistry under the supervision of Prof. Sukh Dev. He worked as Research Associate at Rice University, Houston, USA and as a postdoctoral fellow at the University of Wisconsin, USA between 1977-1980. He returned to India and joined National Chemical Laboratories as Scientist (1980-1986) and started work on pheromones and fatty acids. In 1986, he moved to the Regional Research Laboratory, Hyderabad (presently known as CSIR- Indian Institute of Chemical Technology) and initiated a new era in the field of synthesis. He became the Director in 2003 and headed the crown institute of CSIR (CSIR-IICT) for 9 years (2003-2012). The scientific career of Dr. -

Centers Booklet
THE INSTITUTION The Institute for Social and Economic Change was established in 1972 by the visionary, scholar, statesman and a distinguished social scientist Prof. V K R V Rao. It has always been conceived as an all-India institute of interdisciplinary research and training in social sciences. The principal objective of the Institute is conducting research, pure and applied, in social sciences and enlarging the canvas to include doctoral programme, training and policy dissemination. The main objectives of the Institute summarily stated are to: ● undertake pure and applied research in social sciences encompassing all aspects of a developing society in an inter- disciplinary framework; ● assist central, state and local governments by providing advice based on policy-related studies; ● undertake regular training of doctoral students and teachers in universities and colleges, and for the officials and political functionaries, particularly at local levels; ● maintain a documentation and data centre in social sciences; ● take all measures within the competence of the Institute for the promotion of pure and applied research in social and economic development and change; ● assist the multilateral organisations such as the World Bank, Asian Development Bank, UNFPA , UNICEF, WHO and ILO in undertaking research and training programmes and ● collaborate with universities, research institutions and the non- governmental organisations (NGO) in the region in improving the quality of research and training. CENTRE FOR STUDY OF SOCIAL CHANGE AND DEVELOPMENT (CSSCD) The Centre for Study of Social Change and sociology, they have lately been Development (CSSCD) has been carved out of bypassed in the pursuit of the erstwhile Sociology Unit which was founded understanding change and by none other than Professor M N Srinivas, the development in other arenas. -

Nepal Living Standards Survey 2010/11
NEPAL LIVING STANDARDS SURVEY 2010/11 STATISTICAL REPORT VOLUME TWO CENTRAL BUREAU OF STATISTICS National Planning Commission Secretariat Government of Nepal November 2011 NEPAL LIVING STANDARDS SURVEY 2010/11 STATISTICAL REPORT VOLUME TWO CENTRAL BUREAU OF STATISTICS National Planning Commission Secretariat Government of Nepal November 2011 i Published by Central Bureau of Statistics Thapathali, Kathmandu Nepal Phone: 4229406, 4261506, 4245946-48 Fax: 977-1-4227720 E-mail: [email protected] Web site : www.cbs.gov.np First Edition: November 2011 1,500 copies Printed in Nepal ii iii iv PREFACE It is my pleasure to present the main results of the third Nepal Living Standards Survey (NLSS III). NLSS III is a nation-wide household survey conducted year round through February 2010 to February 2011 which consists of multiple topics related to household welfare. The last Living Standards Survey (NLSS II) was conducted in 2003/04 which updated the poverty profile of the country prepared originally on the basis of the information provided by the first Living Standards Survey (NLSS I) conducted in 1995/96. The present survey has been conducted at a time when political and economic changes are taking place in the country. The survey results are presented in two statistical volumes covering twenty sections of the household questionnaire. The re-introduction of the anthropometric section in the household questionnaire in NLSS III and its successful implementation has provided us new opportunities of multidimensional analysis of the survey data. In fact, the successful completion of the survey has elevated the survey capacity of the CBS in designing, operation, data management and analysis as well. -

How Does Social Protection Contribute to Social Inclusion in Nepal?
February 2014 Report How does social protection contribute to social inclusion in Nepal? Evidence from the Child Grant in the Karnali Region Tej Prasad Adhikari1, Fatik Bahadur Thapa1, Sonam Tamrakar1, Prakash Buda Magar1, Jessica Hagen-Zanker2 and Babken Babajanian2 1NEPAN 2ODI This study uses a social exclusion lens to analyse the effects of Nepal’s Child Grant in Karnali region and tests assumptions about the role social protection can play in contributing to social inclusion and poverty reduction. The study used mixed methods and employed a quasi-experimental impact evaluation. The findings show that in the first three years of implementation, the Child Grant has had only small effects on some indicators of social inclusion, most notably access to a more diversified diet. The research suggests that the impact of the Child Grant is limited by both design and implementation bottlenecks. Shaping policy for development odi.org Preface This report is part of a wider research project that assessed the effectiveness and relevance of social protection and labour programmes in promoting social inclusion in South Asia. The research was undertaken in collaboration with partner organisations in four countries, examining BRAC’s life skills education and livelihoods trainings for young women in Afghanistan, the Chars Livelihoods Programme and the Vulnerable Group Development Programme in Bangladesh, India’s National Health Insurance Programme (RSBY) in Maharashtra and Uttar Pradesh and the Child Grant in the Karnali region of Nepal. Reports and briefings for each country and a paper providing cross-country analysis and drawing out lessons of relevance for regional and international policy can be found at: www.odi.org/sp-inclusion. -

Telling Pacific Lives
TELLING PACIFIC LIVES PRISMS OF PROCESS TELLING PACIFIC LIVES PRISMS OF PROCESS Brij V. Lal & Vicki Luker Editors Published by ANU E Press The Australian National University Canberra ACT 0200, Australia Email: [email protected] This title is also available online at: http://epress.anu.edu.au/tpl_citation.html National Library of Australia Cataloguing-in-Publication entry Title: Telling Pacific lives : prisms of process / editors, Vicki Luker ; Brij V. Lal. ISBN: 9781921313813 (pbk.) 9781921313820 (pdf) Notes: Includes index. Subjects: Islands of the Pacific--Biography. Islands of the Pacific--Anecdotes. Islands of the Pacific--Civilization. Islands of the Pacific--Social life and customs. Other Authors/Contributors: Luker, Vicki. Lal, Brij. Dewey Number: 990.0099 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying or otherwise, without the prior permission of the publisher. Cover design by Teresa Prowse Cover image: Choris, Louis, 1795-1828. Iles Radak [picture] [Paris : s.n., [1827] 1 print : lithograph, hand col.; 20.5 x 26 cm. nla.pic-an10412525 National Library of Australia Printed by University Printing Services, ANU This edition © 2008 ANU E Press Table of Contents Preface vii 1. Telling Pacic Lives: From Archetype to Icon, Niel Gunson 1 2. The Kila Wari Stories: Framing a Life and Preserving a Cosmology, Deborah Van Heekeren 15 3. From ‘My Story’ to ‘The Story of Myself’—Colonial Transformations of Personal Narratives among the Motu-Koita of Papua New Guinea, Michael Goddard 35 4. Mobility, Modernisation and Agency: The Life Story of John Kikang from Papua New Guinea, Wolfgang Kempf 51 5. -
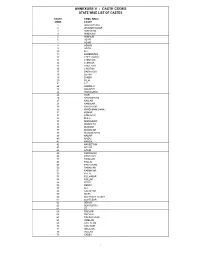
Annexure V - Caste Codes State Wise List of Castes
ANNEXURE V - CASTE CODES STATE WISE LIST OF CASTES STATE TAMIL NADU CODE CASTE 1 ADDI DIRVISA 2 AKAMOW DOOR 3 AMBACAM 4 AMBALAM 5 AMBALM 6 ASARI 7 ASARI 8 ASOOY 9 ASRAI 10 B.C. 11 BARBER/NAI 12 CHEETAMDR 13 CHELTIAN 14 CHETIAR 15 CHETTIAR 16 CRISTAN 17 DADA ACHI 18 DEYAR 19 DHOBY 20 DILAI 21 F.C. 22 GOMOLU 23 GOUNDEL 24 HARIAGENS 25 IYAR 26 KADAMBRAM 27 KALLAR 28 KAMALAR 29 KANDYADR 30 KIRISHMAM VAHAJ 31 KONAR 32 KONAVAR 33 M.B.C. 34 MANIGAICR 35 MOOPPAR 36 MUDDIM 37 MUNALIAR 38 MUSLIM/SAYD 39 NADAR 40 NAIDU 41 NANDA 42 NAVEETHM 43 NAYAR 44 OTHEI 45 PADAIACHI 46 PADAYCHI 47 PAINGAM 48 PALLAI 49 PANTARAM 50 PARAIYAR 51 PARMYIAR 52 PILLAI 53 PILLAIMOR 54 POLLAR 55 PR/SC 56 REDDY 57 S.C. 58 SACHIYAR 59 SC/PL 60 SCHEDULE CASTE 61 SCHTLEAR 62 SERVA 63 SOWRSTRA 64 ST 65 THEVAR 66 THEVAR 67 TSHIMA MIAR 68 UMBLAR 69 VALLALAM 70 VAN NAIR 71 VELALAR 72 VELLAR 73 YADEV 1 STATE WISE LIST OF CASTES STATE MADHYA PRADESH CODE CASTE 1 ADIWARI 2 AHIR 3 ANJARI 4 BABA 5 BADAI (KHATI, CARPENTER) 6 BAMAM 7 BANGALI 8 BANIA 9 BANJARA 10 BANJI 11 BASADE 12 BASOD 13 BHAINA 14 BHARUD 15 BHIL 16 BHUNJWA 17 BRAHMIN 18 CHAMAN 19 CHAWHAN 20 CHIPA 21 DARJI (TAILOR) 22 DHANVAR 23 DHIMER 24 DHOBI 25 DHOBI (WASHERMAN) 26 GADA 27 GADARIA 28 GAHATRA 29 GARA 30 GOAD 31 GUJAR 32 GUPTA 33 GUVATI 34 HARJAN 35 JAIN 36 JAISWAL 37 JASODI 38 JHHIMMER 39 JULAHA 40 KACHHI 41 KAHAR 42 KAHI 43 KALAR 44 KALI 45 KALRA 46 KANOJIA 47 KATNATAM 48 KEWAMKAT 49 KEWET 50 KOL 51 KSHTRIYA 52 KUMBHI 53 KUMHAR (POTTER) 54 KUMRAWAT 55 KUNVAL 56 KURMA 57 KURMI 58 KUSHWAHA 59 LODHI 60 LULAR 61 MAJHE -

The Oaxacan Kitchen the Joy of Cooking Lebanon Italian
EPISODE 301 EPISODE 306 THE OAXACAN KITCHEN CHICKEN AROUND THE WORLD Carnitas Filipino Chicken Adobo with Coconut Broth Green Chili and Tomatillo Crispy Chicken under a Brick Hot Sauce (Tsitsila Tabaka) Tlayudas Chicken Traybake with Roasted Poblano and Tomato Salsa EPISODE 302 EPISODE 307 THE JOY OF COOKING LEBANON GREECE EVERY DAY Za'atar Flatbreads Greek White Bean Soup Pita and Chickpea Salad with (Fasolada) Yogurt and Mint (Fatteh) Spicy Feta Dip (Tirokafteri) Middle Eastern Rice with and Tzatziki Toasted Pasta and Herbs Shrimp with Feta Cheese (Garides Saganaki) EPISODE 303 EPISODE 308 ITALIAN THE RIGHT WAY MILK STREET ITALIAN CLASSICS Risotto with Fresh Herbs Tuscan Beef and Black Pepper Spaghetti al Limone Stew (Peposo alla Fornacina) Pasta all'Amatriciana Pasta con Fagioli Soft Polenta EPISODE 304 EPISODE 309 BAKING IN PARIS ENCHILADAS AND MOLLETES Lemon-Almond Pound Cake Green Enchiladas with Chicken Salted Peanut and Caramel and Cheese (Enchiladas Verdes) Tart Molletes with Pico de Gallo EPISODE 305 EPISODE 310 THE SECRETS OF STIR-FRY ON THE ROAD: PORTLAND, MAINE Vietnamese Shaking Beef (Bò Lúc Lắc) Eventide Green Salad with Nori Vinaigrette Stir-Fried Broccoli with Sichuan Peppercorns Maple–Browned Butter Pie Sesame Stir-Fried Pork with Shiitakes EPISODE 311 EPISODE 316 MILK STREET SWEETS AMAZING ONE-LAYER CAKES Maple-Whiskey Pudding Caprese Chocolate and Cakes Almond Torte Chocolate-Almond Spice Spanish Almond Cake (Tarta Cookies de Santiago) Triple Ginger Scones with Chocolate Chunks Browned Butter and Coconut Loaf Cake -

Pulses Are Praised for Their Health, Environmental and Economic Benefits
PROCEEDINGS Discussion No. 128 30.05.2016 – 19.06.2016 www.fao.org/fsnforum/activities/discussions/pulses Pulses are praised for their health, environmental and economic benefits. How can their full potential be tapped? Collection of contributions received in collaboration with Global Forum on Food Security and Nutrition www.fao.org/fsnforum 2 Pulses are praised for their health, environmental and economic benefits. How can their full potential be tapped PROCEEDINGS Table of Contents Topic note .......................................................................................................................................................................................... 4 Contributions received ................................................................................................................................................................ 6 1. Manuel Castrillo, Proyecto Camino Verde, Costa Rica ...................................................................................... 6 2. Antonia Trichopoulou, Hellenic Health Foundation, Greece ......................................................................... 7 3. Asnake Fikre, Ethiopian Institute of Agricultural Research, Ethiopia ....................................................... 7 4. Kadambot Siddique, UN FAO Special Ambassador for the International Year of Pulses 2016, Australia .......................................................................................................................................................................................