Alaska's Wildlife and Habitat
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glacial Change on Baranof Island: Quantifying Local-Level Impact of Climate Change
Glacial Change on Baranof Island: Quantifying Local-level Impact of Climate Change Jonathan Kreiss-Tomkins, Chandler Kemp, Eli Bildner Overview The glaciers of Baranof Island – the only glaciated island in Southeast Alaska – are small, disparate, and sensitive to climatic change due to the temperate climate in which they are situated. We propose to quantify the change in area of a selection of Baranof Island glaciers over recent history by gathering geospatial data, calculating the perimeter and surface area of the glaciers, using a model to estimate glacial volume, and then comparing our findings against the historical record – historical USGS field measurements, historical aerial photographs, tree core data, and geomorphological indicators such as terminal moraines and trim lines. We will then quantify historical change of surface area and perimeter, and if sufficient historical data points are available, we will also calculate a rate of change (both for surface area and extent of the terminus) and predict future glacial advance or retreat. Methodology Targeted Glaciers We will gather data for two subsets of glaciers, one subset from mid-Baranof Island, one subset from the South Baranof Wilderness Area. The first subset of glaciers will consist of two glaciers from mid-Baranof Island, both on or near the Cross-Baranof Island Trail and well known by users of the Sitka Community Use Area. These glaciers are indicated in map attachment 1 – a small valley glacier north of Glacier Lake and a modest icefield north of the Baranof River valley. The second subset of glaciers will consist of three smaller hanging and cirque glaciers from the South Baranof Wilderness Area (see map attachment 2). -

Bibliography of Alaskan Geology
BIBLIOGRAPHY OF ALASKAN GEOLOGY , ,. SPECIAL REPORT 22 ..... Compiled by: CRAWFORD E. FRITTS and MILDRED E. BROWN State of Alaska Department of Hat ural Resources OIVISIOH OF GEOLOGICAL SURVEY College, Alaska 187 1 STATE OF ALASKA William A. Egan - Governor DEPARTMENT OF NATURAL RESOURCES Charles F. Herbert - Commissioner DIVISION OF GEOLOGICAL SURVEY William C. Fackler - Assistant Commissioner for Minerals BIBLIOGWHY OF ALASKAN GEOLOGY, 1919-1949 Compiled by Crawford E. Fritrs and Mildred E. Brown College, Alaska 1971 CONTENTS Page Introduction ................................ 1 Purpose. source and format .......,............... 1 Serial publications ........................... 2 Other publishing media .........................ll Miscellaneous abbreviations ....................... 13 Bibliography ................................ 15 Index .................. Arealgeology ............. Earthquakes .............. Economic geology ........... Engineering geology .......... General subjects ........... Geomorphology [or physiography] .... Geophysical surveys .......... Glacial geology ............ Historical geology .......... Maps. geologic ............ Mineralogy .............. Paleoclimatology ........... Paleontology ............. Petrology ............... Physical geology ........... Sedimentation or sedimentary petrology Stratigraphy ............. Structural geology .......... Volcanism and volcanology ....... ILLUSTRATIONS Figure 1 . Quadrangles and major geographic divisions of Alaska referred to in this report ....................... -

Robert C. Rhew Dept
Curriculum vitae: June, 2016 Robert Rhew, UC Berkeley Robert C. Rhew Dept. of Geography / Dept. of Environmental Science, Policy and Management Lab: (510) 643-6984 University of California, Berkeley Facsimile: (510) 642-3370 Berkeley, CA 94720-4740 E-mail: [email protected] EDUCATION 1992 B.A. Earth & Planetary Sciences (Atmospheres and Oceans) Harvard University Magna cum laUde with highest honors Cambridge, MA 1994 Graduate Diploma. Resource & Env’t Management Australian National University Diploma with Distinction Canberra, ACT 2001 Ph.D. Earth Sciences (Geochemistry) Scripps Institution of Oceanography, UCSD Thesis title: Production and Consumption of Methyl Bromide and Methyl La Jolla, CA Chloride by the Terrestrial Biosphere ACADEMIC EMPLOYMENT 2012 – present Associate Professor, Dept. Envt. Science, Policy & Mgt. University of California, Berkeley 2009 – present Associate Professor, Department of Geography University of California, Berkeley 2006 – present Geological Scientist Faculty, Earth Sciences Division Lawrence Berkeley National Labs, CA 2013 – 2014 Visiting Faculty Fellow, National Center for Atmospheric Research NCAR, Boulder, CO 2013 – 2014 CIRES Visiting Fellow, NOAA/ CIRES University of Colorado, Boulder 2003 – 2009 Assistant Professor, Department of Geography University of California, Berkeley 2001 – 2003 Postdoctoral Researcher, Earth System Science University of California, Irvine UCAR/NOAA Climate and Global Change Postdoctoral Fellowship, Host: Prof. Eric Saltzman Other appointments: Affiliate faculty in Energy -

Characterization of Moose Movement Patterns and Movement of Black Bears in Relation to Anthropogenic Food Sources on Joint Basse
Form Approved REPORT DOCUMENTATION PAGE OMB No. 074-0188 AD_________________ (Leave blank) Award Number(s): W81XWH-08-2-0179-0002 W912DY-09-2-0011 W9126G-10-2-0042 TITLE: Characterization of moose movement patterns and movement of black bears in relation to anthropogenic food sources on Joint Base Elmendorf- Richardson, Alaska PRINCIPAL INVESTIGATOR: (Enter the name and degree of Principal Investigator and any Associates) Sean D. Farley,Ph.D.; Perry Barboza, Ph.D ; Herman Griese, MS; Christopher Garner, BS CONTRACTING ORGANIZATION: 673d Civil Engineering Squadron 724 Quartermaster Drive Joint Base Elmendorf Richardson, Alaska 99505-8860 REPORT DATE: Sept 2014 TYPE OF REPORT: Final PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland 21702-5012 (W81XWH-08-2-0179-0002) Army Corps of Engineers U.S. Army Engineering and Support Center Huntsville, Alabama 35816-1822 (W912DY-09-2-0011) Army Corps of Engineers U.S. Army Engineering and Support Center Fort Worth, Texas 76102-0300 (W9126G-10-2-0042) DISTRIBUTION STATEMENT: (Check one) X Approved for public release; distribution unlimited Distribution limited to U.S. Government agencies only; report contains proprietary information The views, opinions and/or findings contained in this report are those of the author(s) and should not be construed as an official Department of the Army position, policy or decision unless so designated by other documentation. 1 Public reporting burden for this collection of information is estimated to average 1 hour per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing this collection of information. -

Wildlife Protection Guidelines for Alaska
Wildlife Protection Guidelines for Alaska Alaska Regional Response Team, Wildlife Protection Committee Revision 5 – August 2012 2018 Administrative Update Revision 5 – August 2012 Administrative Update: March 2018 1 Table of Contents I. Introduction ........................................................................................................................... G-5 A. Background G-5 B. Objectives ........................................................................................................................... G-5 C. Scope of Wildlife Protection Guidelines for Alaska ............................................................... G-6 1. Geographic Area ............................................................................................................. G-6 2. Wildlife Resources .......................................................................................................... G-8 3. Wildlife Resource Agencies ............................................................................................. G-8 D. Committee Organization and Development of Guidelines ................................................... G-8 1. Committee Organization ................................................................................................. G-8 2. Development of Guidelines ............................................................................................ G-9 E. Relationship to National Planning Requirements and Guidance .......................................... G-9 F. Procedures for Revisions and -

Biological Monitoring at Aiktak Island, Alaska in 2016
AMNWR 2017/02 BIOLOGICAL MONITORING AT AIKTAK ISLAND, ALASKA IN 2016 Sarah M. Youngren, Daniel C. Rapp, and Nora A. Rojek Key words: Aiktak Island, Alaska, Aleutian Islands, ancient murrelet, Cepphus columba, common murre, double-crested cormorant, fork-tailed storm-petrel, Fratercula cirrhata, Fratercula corniculata, glaucous-winged gull, horned puffin, Larus glaucescens, Leach’s storm-petrel, Oceanodroma furcata, Oceanodroma leucorhoa, pelagic cormorant, Phalacrocorax auritus, Phalacrocorax pelagicus, Phalacrocorax urile, pigeon guillemot, population trends, productivity, red-faced cormorant, Synthliboramphus antiquus, thick-billed murre, tufted puffin, Uria aalge, Uria lomvia. U.S. Fish and Wildlife Service Alaska Maritime National Wildlife Refuge 95 Sterling Highway, Suite 1 Homer, AK 99603 January 2017 Cite as: Youngren, S. M., D. C. Rapp, and N. A. Rojek. 2017. Biological monitoring at Aiktak Island, Alaska in 2016. U.S. Fish and Wildl. Serv. Rep., AMNWR 2017/02. Homer, Alaska. Tufted puffins flying along the southern coast of Aiktak Island, Alaska. TABLE OF CONTENTS Page INTRODUCTION ........................................................................................................................................... 1 STUDY AREA ............................................................................................................................................... 1 METHODS ................................................................................................................................................... -

Moose Hunters in - Southwest Alaska a Better Opportunity to Evaluate Antlers
280 AN EVALUATION OF TROPHY MOOSE MANAGEMENT ON THE ALASKA PENINSULA Christian A. Smith, Alaska Dept. of Fish and Game, King Salmon, Alaska James B. Faro, Alaska Dept. of Fish and Game, Anchorage, Alaska Nicholas C. Steen, Alaska Dept. of Fish and Game, King Salmon, Alaska '" Abstract: an experimental trophy management program was initiated on the Alaska Peninsula in 1976 with the imple mentation of a regulation requiring that all harvested bull moose (AZaes aZaes gigas) have antlers with at least a 50 inch spread. The regulation was designed to protect bulls under 5 years of age, to test the capability of hunters to comply with minimum size requirements, and to determine the potential for maintaining trophy class bulls in the population through this approach. The first two objectives have been accomplished. Nearly 70 - percent of the harvested bulls have been 5 or more years old and only 4 percent of the bulls taken were illegal. Adequate survey data are not available to determine current proportions of trophy bulls in the herd. In view of the declining nature of the population and increasing frequency - of 5 year olds in the kill, however, it seems likely that current harvests may be curtailing recruitment beyond age 5. Although this may not further affect average trophy size, availability of trophy class animals could eventually be - limited to the size of the 5 year old cohort. The moose population of the central Alaska Peninsula, Game Management - Unit 9E, appears to have established via i11111igration southwest from the Naknek River drainage in the early 1930's (Faro 1969). -

Introduction
AMNWR 08/13 BIOLOGICAL MONITORING AT AIKTAK ISLAND, ALASKA IN 2008: SUMMARY APPENDICES Brie A. Drummond Key words: Aiktak Island, Alaska, Aleutian Islands, ancient murrelet, Cepphus columba, common murre, double-crested cormorant, fork-tailed storm-petrel, Fratercula cirrhata, Fratercula corniculata, glaucous-winged gull, horned puffin, Larus glaucescens, Leach’s storm-petrel, Oceanodroma leucorhoa, Oceanodroma furcata, pelagic cormorant, Phalacrocorax auritus, Phalacrocorax pelagicus, Phalacrocorax urile, pigeon guillemot, population trends, productivity, red-faced cormorant, Synthliboramphus antiquus, thick-billed murre, tufted puffin, Uria aalge, Uria lomvia U.S. Fish and Wildlife Service Alaska Maritime National Wildlife Refuge Aleutian Islands Unit 95 Sterling Hwy, Suite 1 Homer, Alaska 99603 September 2008 Cite as: Drummond, B. A. Biological monitoring at Aiktak Island, Alaska in 2008: summary appendices. U.S. Fish and Wildl. Serv. Rep., AMNWR 08/13. Homer, Alaska. 139 pp. Southern coast of Aiktak Island, Alaska, in early June. TABLE OF CONTENTS PAGE INTRODUCTION.......................................................................................................................................... 1 STUDY AREA............................................................................................................................................. ..1 METHODS.................................................................................................................................................. ..2 INTERESTING OBSERVATIONS.............................................................................................................. -

NSF 03-021, Arctic Research in the United States
This document has been archived. Home is Where the Habitat is An Ecosystem Foundation for Wildlife Distribution and Behavior This article was prepared The lands and near-shore waters of Alaska remaining from recent geomorphic activities such by Page Spencer, stretch from 48° to 68° north latitude and from 130° as glaciers, floods, and volcanic eruptions.* National Park Service, west to 175° east longitude. The immense size of Ecosystems in Alaska are spread out along Anchorage, Alaska; Alaska is frequently portrayed through its super- three major bioclimatic gradients, represented by Gregory Nowacki, USDA Forest Service; Michael imposition on the continental U.S., stretching from the factors of climate (temperature and precipita- Fleming, U.S. Geological Georgia to California and from Minnesota to tion), vegetation (forested to non-forested), and Survey; Terry Brock, Texas. Within Alaska’s broad geographic extent disturbance regime. When the 32 ecoregions are USDA Forest Service there are widely diverse ecosystems, including arrayed along these gradients, eight large group- (retired); and Torre Arctic deserts, rainforests, boreal forests, alpine ings, or ecological divisions, emerge. In this paper Jorgenson, ABR, Inc. tundra, and impenetrable shrub thickets. This land we describe the eight ecological divisions, with is shaped by storms and waves driven across 8000 details from their component ecoregions and rep- miles of the Pacific Ocean, by huge river systems, resentative photos. by wildfire and permafrost, by volcanoes in the Ecosystem structures and environmental Ring of Fire where the Pacific plate dives beneath processes largely dictate the distribution and the North American plate, by frequent earth- behavior of wildlife species. -
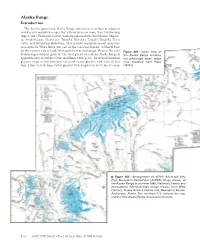
Alaska Range
Alaska Range Introduction The heavily glacierized Alaska Range consists of a number of adjacent and discrete mountain ranges that extend in an arc more than 750 km long (figs. 1, 381). From east to west, named ranges include the Nutzotin, Mentas- ta, Amphitheater, Clearwater, Tokosha, Kichatna, Teocalli, Tordrillo, Terra Cotta, and Revelation Mountains. This arcuate mountain massif spans the area from the White River, just east of the Canadian Border, to Merrill Pass on the western side of Cook Inlet southwest of Anchorage. Many of the indi- Figure 381.—Index map of vidual ranges support glaciers. The total glacier area of the Alaska Range is the Alaska Range showing 2 approximately 13,900 km (Post and Meier, 1980, p. 45). Its several thousand the glacierized areas. Index glaciers range in size from tiny unnamed cirque glaciers with areas of less map modified from Field than 1 km2 to very large valley glaciers with lengths up to 76 km (Denton (1975a). Figure 382.—Enlargement of NOAA Advanced Very High Resolution Radiometer (AVHRR) image mosaic of the Alaska Range in summer 1995. National Oceanic and Atmospheric Administration image mosaic from Mike Fleming, Alaska Science Center, U.S. Geological Survey, Anchorage, Alaska. The numbers 1–5 indicate the seg- ments of the Alaska Range discussed in the text. K406 SATELLITE IMAGE ATLAS OF GLACIERS OF THE WORLD and Field, 1975a, p. 575) and areas of greater than 500 km2. Alaska Range glaciers extend in elevation from above 6,000 m, near the summit of Mount McKinley, to slightly more than 100 m above sea level at Capps and Triumvi- rate Glaciers in the southwestern part of the range. -

Aberrant Plumages in Grebes Podicipedidae
André Konter Aberrant plumages in grebes Podicipedidae An analysis of albinism, leucism, brown and other aberrations in all grebe species worldwide Aberrant plumages in grebes Podicipedidae in grebes plumages Aberrant Ferrantia André Konter Travaux scientifiques du Musée national d'histoire naturelle Luxembourg www.mnhn.lu 72 2015 Ferrantia 72 2015 2015 72 Ferrantia est une revue publiée à intervalles non réguliers par le Musée national d’histoire naturelle à Luxembourg. Elle fait suite, avec la même tomaison, aux TRAVAUX SCIENTIFIQUES DU MUSÉE NATIONAL D’HISTOIRE NATURELLE DE LUXEMBOURG parus entre 1981 et 1999. Comité de rédaction: Eric Buttini Guy Colling Edmée Engel Thierry Helminger Mise en page: Romain Bei Design: Thierry Helminger Prix du volume: 15 € Rédaction: Échange: Musée national d’histoire naturelle Exchange MNHN Rédaction Ferrantia c/o Musée national d’histoire naturelle 25, rue Münster 25, rue Münster L-2160 Luxembourg L-2160 Luxembourg Tél +352 46 22 33 - 1 Tél +352 46 22 33 - 1 Fax +352 46 38 48 Fax +352 46 38 48 Internet: http://www.mnhn.lu/ferrantia/ Internet: http://www.mnhn.lu/ferrantia/exchange email: [email protected] email: [email protected] Page de couverture: 1. Great Crested Grebe, Lake IJssel, Netherlands, April 2002 (PCRcr200303303), photo A. Konter. 2. Red-necked Grebe, Tunkwa Lake, British Columbia, Canada, 2006 (PGRho200501022), photo K. T. Karlson. 3. Great Crested Grebe, Rotterdam-IJsselmonde, Netherlands, August 2006 (PCRcr200602012), photo C. van Rijswik. Citation: André Konter 2015. - Aberrant plumages in grebes Podicipedidae - An analysis of albinism, leucism, brown and other aberrations in all grebe species worldwide. Ferrantia 72, Musée national d’histoire naturelle, Luxembourg, 206 p. -

Recent Changes in the Zooplankton Communities of Arctic Tundra
University of Texas at El Paso DigitalCommons@UTEP Open Access Theses & Dissertations 2019-01-01 Recent Changes In The Zooplankton Communities Of Arctic Tundra Ponds In Response To Warmer Temperatures And Nutrient Enrichment Mariana Vargas Medrano University of Texas at El Paso Follow this and additional works at: https://digitalcommons.utep.edu/open_etd Part of the Biology Commons Recommended Citation Vargas Medrano, Mariana, "Recent Changes In The Zooplankton Communities Of Arctic Tundra Ponds In Response To Warmer Temperatures And Nutrient Enrichment" (2019). Open Access Theses & Dissertations. 2018. https://digitalcommons.utep.edu/open_etd/2018 This is brought to you for free and open access by DigitalCommons@UTEP. It has been accepted for inclusion in Open Access Theses & Dissertations by an authorized administrator of DigitalCommons@UTEP. For more information, please contact [email protected]. RECENT CHANGES IN THE ZOOPLANKTON COMMUNITIES OF ARCTIC TUNDRA PONDS IN RESPONSE TO WARMER TEMPERATURES AND NUTRIENT ENRICHMENT MARIANA VARGAS MEDRANO Doctoral Program in Ecology and Evolutionary Biology APPROVED: Vanessa L. Lougheed, Ph.D., Chair Craig Tweedie, Ph.D. Wen-Yen Lee, Ph.D. Elizabeth J.Walsh, Ph.D. Stephen L. Crites, Jr., Ph.D. Dean of the Graduate School Copyright © by Mariana Vargas Medrano 2019 Dedication This dissertation is dedicated to all my incredible family for all the love and support. RECENT CHANGES IN THE ZOOPLANKTON COMMUNITIES OF ARCTIC TUNDRA PONDS IN RESPONSE TO WARMER TEMPERATURES AND NUTRIENT ENRICHMENT by MARIANA VARGAS MEDRANO, B.S., M.S. DISSERTATION Presented to the Faculty of the Graduate School of The University of Texas at El Paso in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY DEPARTMENT OF BIOLOGICAL SCIENCES THE UNIVERSITY OF TEXAS AT EL PASO AUGUST 2019 Acknowledgments This study was funded by NSF Polar Programs (ARC-0909502).