Fosetyl-Aluminium (302) Phosphonic Acid (301)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

This PDF Was Created from the British Library's Microfilm Copy of The
IMAGING SERVICES NORTH Boston Spa, Wetherby West Yorkshire, LS23 7BQ www.bl.uk This PDF was created from the British Library’s microfilm copy of the original thesis. As such the images are greyscale and no colour was captured. Due to the scanning process, an area greater than the page area is recorded and extraneous details can be captured. This is the best available copy THE BRITISH LIBRARY DOCUMENT SUPPLY CENTRE TITLE Synthesis and Biological Activities of a-aminoalkanephosphonic acids and a-aminoalkanephosphonous acids and their derivatives AUTHOR Fatima Bawa INSTITUTION and DATE The Polytechnic of North London, Attention is drawn to the fact that the copyright of this thesis rests with its author. This copy of the thesis has been supplied on condition that anyone who consults it is understood to recognise that its copyright rests with its author and that no information derived from it may be published without the author’s prior written consent THE BRITISH LIBRARY DOCUMENT SUPPLY CENTRE “ T* ~ n “T4I 1 II 1 II Bocton Spa, W atharty WaitYoffaMr« 20 cmt __L I 1 Unkad Kkifdoin RBOUCnONX'. The Polytechnic of North London in collaboration with Kenogard AB (Sweden) Synthesis and Biological Activities of a-aminoalkanephosphonic acids and a-amlnoalkanephosphonous acids and their derivatives by Fatima Bawa A thesis submitted for the Degree of Doctor of Philosophy of the Council for National Academic Awards. THE BRITISH LIBRARY DOCUMENT SUPPLY CENTRE BRITISH THESES NOT! C E The quality of this reproduction is heavily dependent upon the quality of the original thesis submitted for microfilming. Every effort has been made to ensure the highest quality of reproduction possible. -

Phosphorus and Sulfur Cosmochemistry: Implications for the Origins of Life
Phosphorus and Sulfur Cosmochemistry: Implications for the Origins of Life Item Type text; Electronic Dissertation Authors Pasek, Matthew Adam Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 07/10/2021 06:16:37 Link to Item http://hdl.handle.net/10150/194288 PHOSPHORUS AND SULFUR COSMOCHEMISTRY: IMPLICATIONS FOR THE ORIGINS OF LIFE by Matthew Adam Pasek ________________________ A Dissertation Submitted to the Faculty of the DEPARTMENT OF PLANETARY SCIENCE In Partial Fulfillment of the Requirements For the Degree of DOCTOR OF PHILOSOPHY In the Graduate College UNIVERSITY OF ARIZONA 2 0 0 6 2 THE UNIVERSITY OF ARIZONA GRADUATE COLLEGE As members of the Dissertation Committee, we certify that we have read the dissertation prepared by Matthew Adam Pasek entitled Phosphorus and Sulfur Cosmochemistry: Implications for the Origins of Life and recommend that it be accepted as fulfilling the dissertation requirement for the Degree of Doctor of Philosophy _______________________________________________________________________ Date: 04/11/2006 Dante Lauretta _______________________________________________________________________ Date: 04/11/2006 Timothy Swindle _______________________________________________________________________ Date: 04/11/2006 -
![United States Patent [19] [11] Patent Number: 5,368,832 Buckholtz Et A1](https://docslib.b-cdn.net/cover/1511/united-states-patent-19-11-patent-number-5-368-832-buckholtz-et-a1-641511.webp)
United States Patent [19] [11] Patent Number: 5,368,832 Buckholtz Et A1
USOO5368832A United States Patent [19] [11] Patent Number: 5,368,832 Buckholtz et a1. [45] Date of Patent: Nov. 29, 1994 [54] ZERO DISCI'IARGE PRQCESS FOR 4,330,515 5/1982 Campbell .......................... .. 423/316 MANUFACI'URING OF PHOSPHOROUS 4,380,531 4/1983 Wisnouskas ....................... .. 423/316 ACID AND HYPOPHOSPHOROUS ACID FOREIGN PATENT DOCUMENTS [75] 1I1v¢m°rs= HWY E- Buckh‘?tz, Lewiswn; 0007493 2/1980 European Pat. Off. .......... .. 423/317 Mohan S. Saran, Grand Island, both 254166 11/1986 Japan _ Of N.Y.; Frederick C. Leiterf, 7900920 11/1979 WIPO ............................... .. 423/316 Madison; David A. Flautt, Ashtabula, both of Ohio Primary iExaminer-Michael Lewis Assistant Examiner—-Stephen G. Kalinchak [73] Assignee: gggiggg?gg? Corporation’ gltltglrgey, Agent, or Firm-Wayne A. Jones; Richard D. [21] Appl. No.: 711,841 [57] ' ABS Cr [22] Flled' : Jun . 7 ’ 1991 Dlsclosed. 1s. a method of making. phosphorous acid. or [51] Int. Cl.5 .................. .. C01B 25/165; COlB 25/163 hypophosphomus acid by reacting hydrogen chloride U:S. C1. ................................... .. 423/ 316; 423/ 317 with a sodium phosphite or a sodium hypophosphite’ [58] Field of Search ............. .. 423/ 167, 307, 316, 317, respectively, in the presence of water to precipitate 423/321 R sodium chloride crystals and form the acid. The acid is [56] References Cited separated from the sodium chloride crystals and-can be US PATENT DOCUMENTS passed through amon exchange column that 1s pref erably loaded with phosphite or hypophosphite lons, 2,595,198 4/1952 Leffore et a1. ............ .. 423/316 respectively, to remove'residual chloride ions_ 2,711,388 6/1955 Mottern et a1. -

Phosphorous Acid
Phosphorous acid sc-286667 Material Safety Data Sheet Hazard Alert Code EXTREME HIGH MODERATE LOW Key: Section 1 - CHEMICAL PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME Phosphorous acid STATEMENT OF HAZARDOUS NATURE CONSIDERED A HAZARDOUS SUBSTANCE ACCORDING TO OSHA 29 CFR 1910.1200. NFPA FLAMMABILITY0 HEALTH3 HAZARD INSTABILITY1 SUPPLIER Santa Cruz Biotechnology, Inc. 2145 Delaware Avenue Santa Cruz, California 95060 800.457.3801 or 831.457.3800 EMERGENCY ChemWatch Within the US & Canada: 877-715-9305 Outside the US & Canada: +800 2436 2255 (1-800-CHEMCALL) or call +613 9573 3112 SYNONYMS H3-O3-P, H3PO3, "phosphonic acid", "orthophosphorous acid", "phosphorus trihydroxide", trihydroxyphosphine, "phosphorus acid (sic)", "o-phosphorous acid", "o-phosphorus acid (sic)" Section 2 - HAZARDS IDENTIFICATION CHEMWATCH HAZARD RATINGS Min Max Flammability: 0 Toxicity: 2 Body Contact: 4 Min/Nil=0 Low=1 Reactivity: 1 Moderate=2 High=3 Chronic: 2 Extreme=4 CANADIAN WHMIS SYMBOLS 1 of 9 EMERGENCY OVERVIEW RISK Harmful if swallowed. Causes severe burns. Risk of serious damage to eyes. May cause long-term adverse effects in the aquatic environment. Cumulative effects may result following exposure*. * (limited evidence). POTENTIAL HEALTH EFFECTS ACUTE HEALTH EFFECTS SWALLOWED ■ Accidental ingestion of the material may be harmful; animal experiments indicate that ingestion of less than 150 gram may be fatal or may produce serious damage to the health of the individual. ■ Ingestion of acidic corrosives may produce burns around and in the mouth, the throat and oesophagus. Immediate pain and difficulties in swallowing and speaking may also be evident. ■ As absorption of phosphates from the bowel is poor, poisoning this way is less likely. -

Neutralized Phosphorous Acid to Control Phytophthora Diseases
Plant Pathology Bulletin 18: 155-165, 2009 A Simple Technique, Concentration and Application Schedule for Using Neutralized Phosphorous Acid to Control Phytophthora Diseases Pao-Jen Ann 1, 4, Jyh-Nong Tsai 1, In-Ting Wong 1, Ting-Fang Hsieh 2, and Chien-Yih Lin 3 1. Plant Pathology Division, Taiwan Agriculture Research Institute, Taichung, 413, Taiwan 2. Floriculture Research Center, Taiwan Agriculture Research Institute, Yulin, 646, Taiwan 3. College of Health Science, Asia University, Taichung 413, Taiwan 4. Corresponding author, E-mail: [email protected]; Fax: 886-4-2330-2803 Accepted for publication: September 19, 2009 ABSTRACT Ann, P. J., Tsai, J. N., Wong, I. T., Hsieh, T. F., and Lin, C. Y. 2009. A simple technique, concentration and application schedule for using neutralized phosphorous acid to control Phytophthora diseases. Plant Pathol. Bull. 18: 155-165. A simple method for application of phosphorous acid to control Phytophthora diseases was developed. The proposed method involves measuring equal weight of phosphorous acid and potassium hydroxide and dissolving phosphorous acid in water before adding potassium hydroxide. The pH of the neutralized phosphorous acid solution (NPA) is 6.2-6.7. The NPA concentration at 5000 mg/L was effective in controlling tomato seedling blight caused by Phytophthora capsici when using soil drench method. For suppression of lily leaf and blossom blight caused by Phytophthora parasitica, foliar application is much more effective than soil drench. However, NPA concentrations exceeding 2000 mg/L were phytotoxic to young plant tissues. The best results were achieved by applying NPA at the rate of 1000 mg/L for 3 times at one week intervals. -

United States Patent (19) 11) 4,278,647 Jüdden Et Al
United States Patent (19) 11) 4,278,647 Jüdden et al. 45) Jul. 14, 1981 54 PRODUCTION OF PHOSPHOROUS ACID 56) References Cited 75 Inventors: Klaus Jödden, Hirth; Hans-Werner FOREIGN PATENT DOCUMENTS Stephan, Cologne; Gero Heymer, 1218063 12/1958 France ..................................... 423/317 Erftstadt, all of Fed. Rep. of Primary Examiner-O. R. Vertiz Germany Assistant Examiner-Gregory A. Heller 73) Assignee: Hoechst Aktiengesellschaft, Fed. 57 ABSTRACT Rep. of Germany The invention relates to a process for making aqueous 21 Appl. No.: 60,659 phosphorous acid solutions by admixing an aqueous sodium phosphite solution with hydrochloric acid. 22 Filed: Jul. 25, 1979 More specifically, phosphorous acid solutions substan tially free from sodium in hydrochloric acid are pro (30) Foreign Application Priority Data duced by introducing hydrogen chloride, into the so Jul, 29, 1978 DE Fed. Rep. of Germany ....... 2833380 dium phosphite solution until the latter is saturated therewith and separating precipitating sodium chloride 51 Int. Cl. ....................... C01B 25/16; CO1B 25/26 from the resulting phosphorous acid solution in hydro 52 U.S.C. ..................................... 423/317; 423/307 chloric acid. 58 Field of Search ............... 423/317, 307, 499, 481, 423/316 8 Claims, No Drawings 4,278,647 2 adversely affect the work up of the resulting phospho PRODUCTION OF PHOSPHOROUS ACID rous acid solution, it is good practice, prior to the intro duction of hydrogen chloride, to pass a free oxygen This invention relates to a process for making aque containing gas, e.g. air or oxygen, through the sodium ous phosphorous acid solutions by admixing an aqueous phosphite solution at elevated temperature, preferably sodium phosphite solution with hydrochloric acid. -

Product Safety Summary
Product Safety Summary Phosphorus Trichloride CAS No. 7719-12-2 This Product Safety Summary is intended to provide a general overview of the chemical substance. The information in the summary is basic information and is not intended to provide emergency response information, medical information or treatment information. The summary should not be used to provide in-depth safety and health information. In-depth safety and health information can be found in the Safety Data Sheet (SDS) for the chemical substance. Names Phosphorus trichloride Phosphorus (III) chloride Phosphorous chloride PCl 3 Product Overview Solvay Novecare does not sell phosphorus trichloride directly to consumers and phosphorus trichloride has no known uses except as an intermediate to produce other chemicals. Phosphorus trichloride (PCl3) is used as a chemical intermediate to produce a variety of products which are used in several applications including agricultural products, surfactants and metal extractants, flame retardants, additives for lubricants and stabilizers for plastics. Phosphorus trichloride is used in industrial applications and other processes where workplace exposures can occur. Consumer exposure does not occur as phosphorus trichloride is not used in any commercially available product. Phosphorus trichloride is dangerous to human health. Phosphorus trichloride may be fatal if inhaled, highly toxic if swallowed, harmful if absorbed through skin and can cause severe burns which may result in scarring. Phosphorus trichloride is consumed in manufacturing processes. PCl3 can make its way into the environment through unintentional releases (spills). PCl3 will not bioaccumulate but is not biodegradable. Based on ecotoxicological testing performed on fish and fresh-water invertebrates, PCl3 in higher concentrations can be harmful to aquatic life due to formation of acids from the hydrolysis of PCl3. -

CHEM 1411 Nomenclature Homework - Answers Part I
1 CHEM 1411 Nomenclature Homework - Answers Part I 1. The following are a list of binary and pseudobinary ionic compounds. Write the name when the formula is given. Write the formula when the name is given. (a) AlCl3 aluminum chloride (k) rubidium oxide Rb2O (b) AuBr3 gold (III) bromide (l) chromium (III) selenide Cr2Se3 (c) Na2S sodium sulfide (m) barium iodide BaI2 (d) Cu3P2 copper (II) phosphide (n) copper (I) fluoride CuF (e) Fe(OH)2 iron (II) hydroxide (o) copper (II) fluoride CuF2 (f) NH4OH ammonium hydroxide (p) strontium cyanide Sr(CN)2 (g) Co(CH3COO)3 cobalt (III) acetate (q) mercury (II) bromide HgBr2 (h) Zn(SCN)2 zinc thiocyanate (r) mercury (I) bromide Hg2Br2 (i) CaCrO4 calcium chromate (s) magnesium permanganate Mg(MnO4)2 (j) K2Cr2O7 potassium dichromate (t) lithium nitride Li3N 2. The following are lists of covalent compounds. Write the name when a formula is given. Write the formula when given a name. (a) CSe2 carbon diselenide (h) dichlorine heptoxide Cl2O7 (b) SF6 sulfur hexafluoride (i) xenon tetrafluoride XeF4 (c) BrF5 bromine pentafluoride (j) carbon monoxide CO (d) P4O10 tetraphosphorous decoxide (k) oxygen O2 (e) Cl2O dichlorine oxide (l) diboron trioxide B2B O3 (f) NH3 ammonia (m) arsenic trifluoride AsF3 (g) N2 dinitrogen or nitrogen (n) diiodine I2 2 3. The following are lists of acids or acid-forming compounds. Write the name when the formula is given. Write the formula when the name is given. (a) H3PO2 hypophosphorous acid (k) hydrogen cyanide HCN (g) (b) H2SO4 sulfuric acid (l) periodic acid HIO4 (c) HClO hypochlorous acid (m) hypochlorous acid HClO (d) H3PO4 phosphoric acid (n) nitric acid HNO3 (e) HBrO4 perbromic acid (o) acetic acid CH3CO2H (f) HIO2 iodous acid (p) chloric acid HClO3 (g) HI (g) hydrogen iodide (q) perbromic acid HBrO4 (h) HI (aq) hydroiodic acid (r) hydrofluoric acid HF (aq) (i) HCN (aq) hydrocyanic acid (s) phosphorous acid H3PO3 (j) HBrO hypobromous acid (t) hydrosulfuric acid H2S (aq) 4. -

Junta De Explotación: CENIA-MAESTRAZGO Junta De Explotación: CENIA-MAESTRAZGO Apartado: 41B R.D
Junta de Explotación: CENIA-MAESTRAZGO Junta de Explotación: CENIA-MAESTRAZGO apartado: 41b R.D. 927/1988 < 100.000 habitantes Usuario Municipio Provincia Hab. Servidos AYUNTAMIENTO DE ALCALÀ DE XIVERT Alcalà de Xivert Castellón 12.659 AYUNTAMIENTO DE BENICARLO Benicarló Castellón 34.241 AYUNTAMIENTO DE CABANES Cabanes Castellón 1.315 AYUNTAMIENTO DE CÀLIG Càlig Castellón 2.198 AYUNTAMIENTO DE CANET LO ROIG Vallibona Castellón 3.119 AYUNTAMIENTO DE CATI Catí Castellón 2.147 AYUNTAMIENTO DE CERVERA DEL MAESTRE Cervera del Maestre Castellón 1.099 AYUNTAMIENTO DE CHERT/XERT Chert/Xert Castellón 4.230 AYUNTAMIENTO DE LES COVES DE VINROMA Coves de Vinromà (les) Castellón 2.501 AYUNTAMIENTO DE OROPESA DEL MAR Cabanes Castellón 6.815 AYUNTAMIENTO DE ROSSELL Pobla de Benifassà (la) Castellón 2.003 AYUNTAMIENTO DE SALZADELLA (LA) Salzadella (la) Castellón 1.234 AYUNTAMIENTO DE SAN JORGE Traiguera Castellón 693 AYUNTAMIENTO DE SAN RAFAEL DEL RIO Pobla de Benifassà (la) Castellón 866 AYUNTAMIENTO DE SANT MATEU Sant Mateu Castellón 3.407 AYUNTAMIENTO DE SANTA MAGDALENA DE PULPIS Santa Magdalena de Pulpis Castellón 1.408 AYUNTAMIENTO DE SENIA (LA) Sénia (La) Tarragona 8.418 AYUNTAMIENTO DE SIERRA ENGARCERAN Sierra Engarcerán Castellón 1.308 AYUNTAMIENTO DE TORREBLANCA Torreblanca Castellón 5.512 AYUNTAMIENTO DE TRAIGUERA Traiguera Castellón 390 AYUNTAMIENTO DE ULLDECONA Pobla de Benifassà (la) Castellón 1.501 AYUNTAMIENTO DE VALLIBONA Vallibona Castellón 67 AYUNTAMIENTO DE VILANOVA D`ALCOLEA Vilanova d´Alcolea Castellón 58 AYUNTAMIENTO DE VINARÒS Vinaròs Castellón 38.324 LOGER, S.A. (GRUPO MARINA D´OR) Oropesa del Mar/Orpesa Castellón 8.022 VERDEFEBA, S.A. San Jorge Castellón 10.048 Total: 153.583 Junta de Explotación: CENIA-MAESTRAZGO apartado: 41d R.D. -

United States Patent Office Patented July 29, 1969
3,458,281 United States Patent Office Patented July 29, 1969 2 into phosphoric acid alone, and they generally have a 3,458,281 short life in concentrated phosphorous acid solutions. MANUEFACTURE OF PHOSPHOROUS ACID The conventional method for the manufacture of phos Michel Charles Demarcq, Lyon, France, assignor, by phorous acid comprises hydrolyzing phosphorus trichlo mesne assignments, to Ugine Kuhlmann (Societe Ano nyme), Paris, France, a French corporation ride according to the diagrammatically simplified reaction: No Drawing. Filed Mar. 7, 1966, Ser. No. 532,115 (1) PCls--3HO->HPO-3HCI (Claims priority, application France, Mar. 8, 1965, 8,329 and evaporating the excess water and the hydrogen chlo int. C. CO 25/16 ride which are formed. U.S. C. 23-65 8 Claims Actually traces of phosphorus compounds other than O phosphorous acid, including phosphoric acid are also formed in the course of said hydrolysis. The two main ABSTRACT OF THE DISCLOSUIRE Sources of said compounds are: A method for the production of phosphorous acid sub (a) The lack of purity of the phosphorus trichloride stantially free of phosphorus acids having a degree of 15 used in which traces of phosphorus oxychloride and phos oxidation higher than that of phosphorous acid which phorus pentachloride evidently lead to the formation of comprises the steps of (a) reacting phosphorus trichloride phosphoric acid in the course of the hydrolysis; with from 3 to 6 moles per mole of phosphorus trichloride (b) The spontaneous dismutation of nascent phos of a hydroxylated compound having the formula phorous acid, which leads on one hand to superoxidized 20 phosphorus acids (orthophosphoric, hypophosphoric, iso ROH hypophosphoric, pyrophosphoric acids) and on the other wherein R represents a member selected from the group to underoxidized derivatives (hypophosphorous acids, consisting of alkyl, chloroalkyl, hydroxyalkyl, alkoxyalkyl phosphine etc. -
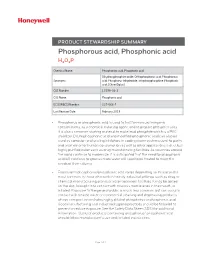
Phosphorous Acid, Phosphonic Acid H3O3P
PRODUCT STEWARDSHIP SUMMARY Phosphorous acid, Phosphonic acid H3O3P Chemical Name: Phosphorous acid, Phosphonic acid Dihydroxyphosphine oxide; Orthophosphorus acid; Phosphorous Synonyms: acid; Phosphorus trihydroxide; Trihydroxyphosphine; Phosphonic acid; [ChemIDplus] CAS Number: 13598-36-2 CAS Name: Phosphonic acid EC (EINECS) Number: 237-066-7 Last Revision Date: February 2019 • Phosphorus or phosphonic acid is used to test for mercury inorganic contaminants, as a chemical reducing agent, and to prepare phosphite salts. It is also a common starting material to make lead phosphite which is a PVC stabilizer. Dilute phosphonic acid and modified phosphonic acid can also be used as corrosion and scaling inhibitors in cooling water systems used to purify and treat water for human consumption as well as other applications that utilize highly purified water such as drug manufacturing facilities. As countries around the world continue to modernize, it is anticipated that the need for phosphonic acid will continue to grow as more water will need to be treated to meet the needs of their citizens. • Exposure to phosphorus/phosphonic acid varies depending on its use and is most common in those who work in heavily industrial settings such as drug or chemical manufacturing plants or water treatment facilities. It may be spilled on the skin, brought into contact with mucous membranes in the mouth, or inhaled. Exposure to the general public is much less common but can occur in contact with treated water or commercial cleaning and degreasing products whose composition includes highly diluted phosphorus or phosphonic acid. Good manufacturing and industrial hygiene practices should be followed to prevent or reduce exposure. -

Red De Refugios, Cabañas Y Campamentos
plazas de la instalación. la de plazas fregaderos… huso 30, datum ED50. datum 30, huso Siempre se deberá abonar la totalidad de las las de totalidad la abonar deberá se Siempre aseos con wc y duchas, zona adaptada para cocinar, cocinar, para adaptada zona duchas, y wc con aseos terreno. Las coordenadas están en UTM en el el en UTM en están coordenadas Las terreno. estancia durante un periodo no superior a 15 días: agua, agua, días: 15 a superior no periodo un durante estancia tat Valenciana. [2008/993] Valenciana. tat mos de mantenimiento de las instalaciones. las de mantenimiento de mos de distancia,Z para la creación de perfiles del del perfiles de creación la para distancia,Z de de lucro. Disponen del equipamiento necesario para la la para necesario equipamiento del Disponen lucro. de - Comuni la de forestales terrenos los por los Precio simbólico para cubrir los gastos míni- gastos los cubrir para simbólico Precio asociaciones o grupos juveniles o sociales sin ánimo ánimo sin sociales o juveniles grupos o asociaciones entre una lista de coordenadas X,Y,Z, o valores valores o X,Y,Z, coordenadas de lista una entre - vehícu de circulación la regula se que el por io? C re p ¿ das de campaña, y que sean utilizados por entidades, entidades, por utilizados sean que y campaña, de das ordenadas correspondientes, pudiendo elegir elegir pudiendo correspondientes, ordenadas el Decreto 8/2008, de 25 de enero, del Consell, Consell, del enero, de 25 de 8/2008, Decreto el - tien con temporal y se rellenará el cuadro de texto con las co- las con texto de cuadro el rellenará se y las pistas forestales, tal y como se recoge en en recoge se como y tal forestales, pistas las para su ocupación ocupación su para En cabañas no excederá de las 6 noches.