Alphabetical Index of Substances and Articles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rhode Island Hazardous Substance List
Rhode Island Hazardous Substance List Source: T - ACGIH F - NFPA49 C - IARC Alphabetical Order C.A.S. ACGIH NFPA IARC CHEMICAL NAME 13010-47-4 C 1,-(2-Chloroethyl)-3-cyclohexyl-1-Nitrosourea 76-11-9 T 1,1,1,2-tetrachloro-2,2-difluoroethane 76-12-0 T 1,1,2,2-tetrachloro-1,2-difluoroethane 79-34-5 T 1,1,2,2-tetrachloroethane - skin 76-13-1 T 1,1,2-trichloro-1,2,2-trifluoroethane 79-00-5 T F C 1,1,2-trichloroethane - skin 594-72-9 T 1,1-Dichloro-1-nitroethane 74-34-3 T 1,1-dichloroethane 57-14-7 T 1,1-dimethylhydrazine (udmh) 96-18-4 T 1,2,3-trichloropropane 120-82-1 T 1,2,4-Trichlorobenzene 106-88-7 F 1,2-Butylene oxide 107-15-3 T F 1,2-Diaminoethane 96-12-8 C 1,2-Dibromo-3-chloropropane 106-93-4 T F C 1,2-Dibromoethane - skin 107-06-2 T F 1,2-Dichlorethane 540-59-0 T F 1,2-Dichloroethene 540-59-0 T F 1,2-Dichloroetylene 1615-80-1 C 1,2-Diethylhydrazine C 1,2-Dimethyl hydrazine - skin 106-99-0 T F 1,3-Butadiene 118-52-5 T 1,3-Dichloro-5,5-dimethylhydantoin 542-75-6 T F 1,3-Dichloropropene (cis and trans) 542-75-6 T F 1,3-Dichloropropylene 110-56-5 F 1,4-Dichlorobutane 123-91-1 T F C 1,4-Dioxane 1120-71-4 1-3-Propane sultone 110-53-2 F 1-Bromopentane 106-89-8 T F C 1-Chloro,2,3-epoxy-propane 600-25-9 T 1-Chloro-1-nitropropane 97-00-7 F 1-chloro-2,4-dinitrobenzene 543-59-9 F 1-Chloropentane 112-30-1 F 1-Decanol 111-27-3 F 1-Hexanol 141-79-7 T F 1-Isobutenyl methyl ketone 108-03-2 T F 1-Nitropropane 71-41-0 F 1-Pentanol 110-58-7 F 1-Pentylamine 111-40-0 T F 2,2'-Diaminodiethylamine 111-44-4 F 2,2'Dichlorodiethyl ether 75-99-0 T 2,2-dichloropropionic acid 556-52-5 T 2,3-Epoxy-1-propanol 93-76-5 T 2,4,5-T 95-95-4 F 2,4,5-trichlorophenol 88-06-2 F C 2,4,6-trichlorophenol 118-96-7 T F 2,4,6-Trinitro Toluene 479-95-8 T 2,4,6-Trinitrophenyl-methylnitramine 94-75-7 T 2,4-d (2,4-dichlorophenoxyacetic acid) 97-02-9 F 2,4-dinitroaniline 584-84-9 T F 2,4-Tolylene diisocyanate 108-83-8 T 2,6-Dimethyl-4-heptanone 108-83-8 T 2,6-Dimethyl-4-heptanone 128-37-0 T 2,6-Ditert. -

1,1,1,2-Tetrafluoroethane
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organisation, or the World Health Organization. Concise International Chemical Assessment Document 11 1,1,1,2-Tetrafluoroethane First draft prepared by Mrs P. Barker and Mr R. Cary, Health and Safety Executive, Liverpool, United Kingdom, and Dr S. Dobson, Institute of Terrestrial Ecology, Huntingdon, United Kingdom Please not that the layout and pagination of this pdf file are not identical to the printed CICAD Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals. World Health Organization Geneva, 1998 The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organisation (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. The Inter-Organization -

SAFETY DATA SHEET Difluoromethane (R32) SECTION 1
SAFETY DATA SHEET Difluoromethane (R32) Issue Date: 16.01.2013 Version: 1.1 SDS No.: 000010021734 Last revised date: 26.11.2018 1/14 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product identifier Product name: Difluoromethane (R32) Other Name: HFC-32 Additional identification Chemical name: Difluoromethane Chemical formula: CH2F2 INDEX No. - CAS-No. 75-10-5 EC No. 200-839-4 REACH Registration No. 01-2119471312-47 1.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses: Industrial and professional. Perform risk assessment prior to use. Refrigerant. Use as an Intermediate (transported, on-site isolated). Use for electronic component manufacture. Using gas alone or in mixtures for the calibration of analysis equipment. Formulation of mixtures with gas in pressure receptacles. Uses advised against Consumer use. 1.3 Details of the supplier of the safety data sheet Supplier Linde Gas GmbH Telephone: +43 50 4273 Carl-von-Linde-Platz 1 A-4651 Stadl-Paura E-mail: [email protected] 1.4 Emergency telephone number: Emergency number Linde: + 43 50 4273 (during business hours), Poisoning Information Center: +43 1 406 43 43 SECTION 2: Hazards identification 2.1 Classification of the substance or mixture Classification according to Regulation (EC) No 1272/2008 as amended. Physical Hazards Flammable gas Category 1 H220: Extremely flammable gas. Gases under pressure Liquefied gas H280: Contains gas under pressure; may explode if heated. SDS_AT - 000010021734 SAFETY DATA SHEET Difluoromethane (R32) Issue Date: 16.01.2013 Version: 1.1 SDS No.: 000010021734 Last revised date: 26.11.2018 2/14 2.2 Label Elements Signal Words: Danger Hazard Statement(s): H220: Extremely flammable gas. -
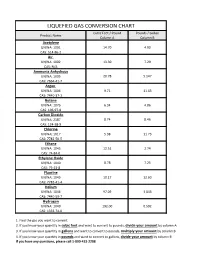
Liquefied Gas Conversion Chart
LIQUEFIED GAS CONVERSION CHART Cubic Feet / Pound Pounds / Gallon Product Name Column A Column B Acetylene UN/NA: 1001 14.70 4.90 CAS: 514-86-2 Air UN/NA: 1002 13.30 7.29 CAS: N/A Ammonia Anhydrous UN/NA: 1005 20.78 5.147 CAS: 7664-41-7 Argon UN/NA: 1006 9.71 11.63 CAS: 7440-37-1 Butane UN/NA: 1075 6.34 4.86 CAS: 106-97-8 Carbon Dioxide UN/NA: 2187 8.74 8.46 CAS: 124-38-9 Chlorine UN/NA: 1017 5.38 11.73 CAS: 7782-50-5 Ethane UN/NA: 1045 12.51 2.74 CAS: 74-84-0 Ethylene Oxide UN/NA: 1040 8.78 7.25 CAS: 75-21-8 Fluorine UN/NA: 1045 10.17 12.60 CAS: 7782-41-4 Helium UN/NA: 1046 97.09 1.043 CAS: 7440-59-7 Hydrogen UN/NA: 1049 192.00 0.592 CAS: 1333-74-0 1. Find the gas you want to convert. 2. If you know your quantity in cubic feet and want to convert to pounds, divide your amount by column A 3. If you know your quantity in gallons and want to convert to pounds, multiply your amount by column B 4. If you know your quantity in pounds and want to convert to gallons, divide your amount by column B If you have any questions, please call 1-800-433-2288 LIQUEFIED GAS CONVERSION CHART Cubic Feet / Pound Pounds / Gallon Product Name Column A Column B Hydrogen Chloride UN/NA: 1050 10.60 8.35 CAS: 7647-01-0 Krypton UN/NA: 1056 4.60 20.15 CAS: 7439-90-9 Methane UN/NA: 1971 23.61 3.55 CAS: 74-82-8 Methyl Bromide UN/NA: 1062 4.03 5.37 CAS: 74-83-9 Neon UN/NA: 1065 19.18 10.07 CAS: 7440-01-9 Mapp Gas UN/NA: 1060 9.20 4.80 CAS: N/A Nitrogen UN/NA: 1066 13.89 6.75 CAS: 7727-37-9 Nitrous Oxide UN/NA: 1070 8.73 6.45 CAS: 10024-97-2 Oxygen UN/NA: 1072 12.05 9.52 CAS: 7782-44-7 Propane UN/NA: 1075 8.45 4.22 CAS: 74-98-6 Sulfur Dioxide UN/NA: 1079 5.94 12.0 CAS: 7446-09-5 Xenon UN/NA: 2036 2.93 25.51 CAS: 7440-63-3 1. -

Gas Conversion Factor for 300 Series
300GasTable Rec # Gas Symbol GCF Density (g/L) Density (g/L) 25° C / 1 atm 0° C / 1 atm 1 Acetic Acid C2H4F2 0.4155 2.7 2.947 2 Acetic Anhydride C4H6O3 0.258 4.173 4.555 3 Acetone C3H6O 0.3556 2.374 2.591 4 Acetonitryl C2H3N 0.5178 1.678 1.832 5 Acetylene C2H2 0.6255 1.064 1.162 6 Air Air 1.0015 1.185 1.293 7 Allene C3H4 0.4514 1.638 1.787 8 Ammonia NH3 0.7807 0.696 0.76 9 Argon Ar 1.4047 1.633 1.782 10 Arsine AsH3 0.7592 3.186 3.478 11 Benzene C6H6 0.3057 3.193 3.485 12 Boron Trichloride BCl3 0.4421 4.789 5.228 13 Boron Triflouride BF3 0.5431 2.772 3.025 14 Bromine Br2 0.8007 6.532 7.13 15 Bromochlorodifluoromethane CBrClF2 0.3684 6.759 7.378 16 Bromodifluoromethane CHBrF2 0.4644 5.351 5.841 17 Bromotrifluormethane CBrF3 0.3943 6.087 6.644 18 Butane C4H10 0.2622 2.376 2.593 19 Butanol C4H10O 0.2406 3.03 3.307 20 Butene C4H8 0.3056 2.293 2.503 21 Carbon Dioxide CO2 0.7526 1.799 1.964 22 Carbon Disulfide CS2 0.616 3.112 3.397 23 Carbon Monoxide CO 1.0012 1.145 1.25 24 Carbon Tetrachloride CCl4 0.3333 6.287 6.863 25 Carbonyl Sulfide COS 0.668 2.456 2.68 26 Chlorine Cl2 0.8451 2.898 3.163 27 Chlorine Trifluoride ClF3 0.4496 3.779 4.125 28 Chlorobenzene C6H5Cl 0.2614 4.601 5.022 29 Chlorodifluoroethane C2H3ClF2 0.3216 4.108 4.484 30 Chloroform CHCl3 0.4192 4.879 5.326 31 Chloropentafluoroethane C2ClF5 0.2437 6.314 6.892 32 Chloropropane C3H7Cl 0.308 3.21 3.504 33 Cisbutene C4H8 0.3004 2.293 2.503 34 Cyanogen C2N2 0.4924 2.127 2.322 35 Cyanogen Chloride ClCN 0.6486 2.513 2.743 36 Cyclobutane C4H8 0.3562 2.293 2.503 37 Cyclopropane C3H6 0.4562 -

160 'Ideal' Gases: Anaesthetics in the Heart of the Twentieth Century Ian
International Workshop on the History of Chemistry 2015 Tokyo ‘Ideal’ Gases: Anaesthetics in the Heart of the Twentieth Century Ian D. Rae University of Melbourne, Australia 1. Introduction By 1920 only three gaseous anaesthetics were widely used – nitrous oxide, diethyl ether (ether) and chloroform. The toxicity of chloroform was acknowledged, nitrous oxide did not induce deep anaesthesia, and ether was extremely inflammable, so in the 1920s there were good reasons to search for new anaesthetics. While my concern is with gaseous anaesthetics, I recognise that there were parallel developments in two related fields, that of topical or local anaesthetics, typified by the natural product cocaine and a host of synthetic substances, and injectable anaesthetics starting with opiates, then barbiturates and leading to modern materials such as propofol (2,6-diisopropylphenol). 2. Theories of anaesthetic action Hans Meyer1 noted that the anaesthetic substances were soluble in both fatty and aqueous media, proposed a general theory of anaesthesia based on the partition or distribution coefficient as a critical determinant. Meyer enunciated the following three principles that underpinned his theory: all chemically inert substances that are soluble in fats and fatty materials will produce narcosis; the line of action is in the nerve cells; the comparative strengths of substances depend on their solubility in fatty material and in water, that is, on the distribution coefficient. Charles Overton arrived at the same idea independently. Some years after completing his PhD research on cell permeability studies, Overton first presented his theory of narcosis in a lecture to the Society for Natural History in Zurich in October 1898, in a paper published the following year2 and in his book3 which included a full exposition. -

Addressing Highly Hazardous Pesticides in Mozambique
República de Moçambique Ministério da Agricultura e Segurança Alimentar Direcção Nacional de Agricultura e Silvicultura Addressing Highly Hazardous Pesticides in Mozambique Addressing Highly Hazardous Pesticides in Mozambique Food and Agriculture Organization of the United Nations Rome, 2016 The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. The views expressed in this information product are those of the author(s) and do not necessarily reflect the views or policies of FAO. ISBN 978-92-5-108519-6 © FAO, 2016 FAO encourages the use, reproduction and dissemination of material in this information product. Except where otherwise indicated, material may be copied, downloaded and printed for private study, research and teaching purposes, or for use in non-commercial products or services, provided that appropriate acknowledgement of FAO as the source and copyright holder is given and that FAO’s endorsement of users’ views, products or services is not implied in any way. All requests for translation and adaptation rights, and for resale and other commercial use rights should be made via www.fao.org/contact-us/licence-request or addressed to [email protected]. -

Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 Theinternational Programme on Chemical Safety (IPCS) Was Established in 1980
The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 cation Hazard of Pesticides by and Guidelines to Classi The WHO Recommended Classi The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 TheInternational Programme on Chemical Safety (IPCS) was established in 1980. The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. This publication was developed in the IOMC context. The contents do not necessarily reflect the views or stated policies of individual IOMC Participating Organizations. The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase international coordination in the field of chemical safety. The Participating Organizations are: FAO, ILO, UNDP, UNEP, UNIDO, UNITAR, WHO, World Bank and OECD. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment. WHO recommended classification of pesticides by hazard and guidelines to classification, 2019 edition ISBN 978-92-4-000566-2 (electronic version) ISBN 978-92-4-000567-9 (print version) ISSN 1684-1042 © World Health Organization 2020 Some rights reserved. -

A Review of Aluminium Phosphide Poisoning and a Flowchart to Treat It Arh Hig Rada Toksikol 2016;67:183-193 183
Hashemi-Domeneh, et al. A review of aluminium phosphide poisoning and a flowchart to treat it Arh Hig Rada Toksikol 2016;67:183-193 183 Review DOI: 10.1515/aiht-2016-67-2784 A review of aluminium phosphide poisoning and a flowchart to treat it Behrooz Hashemi-Domeneh1,2, Nasim Zamani1,2, Hossein Hassanian-Moghaddam1,2, Mitra Rahimi1,2, Shahin Shadnia1,2, Peyman Erfantalab1,2, and Ali Ostadi1,2 Toxicological Research Center, Department of Clinical Toxicology, Loghman-Hakim Hospital, Shahid Beheshti University of Medical Sciences1, Excellence Center of Clinical Toxicology, Iranian Ministry of Health2, Tehran, Iran [Received in February 2016; CrossChecked in February 2016; Accepted in September 2016] The use of pesticides such as aluminium phosphide (AlP) has increased in the recent years and improved the quantity and quality of agricultural products in a number of developing countries. The downside is that AlP causes severe chronic and acute health effects that have reached major proportions in countries such as India, Iran, Bangladesh, and Jordan. Nearly 300,000 people die due to pesticide poisoning in the world every year. Poisoning with AlP accounts for many of these deaths. Unfortunately, at the same time, there is no standard treatment for it. The aim of this article is to give a brief review of AlP poisoning and propose a treatment flowchart based on the knowledge gained so far. For this purpose we reviewed all articles on the management of AlP poisoning published from 2000 till now. Using a modified Delphi design, we have designed a handy flowchart that could be used as a guide for AlP poisoning management of patients in emergency centres. -
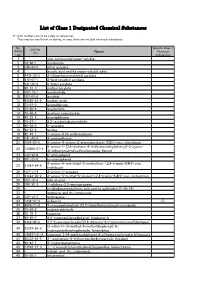
List of Class 1 Designated Chemical Substances
List of Class 1 Designated Chemical Substances *1:CAS numbers are to be solely as references. They may be insufficient or lacking, in case there are multiple chemical substances. No. Specific Class 1 CAS No. (PRTR Chemical (*1) Name Law) Substances 1 - zinc compounds(water-soluble) 2 79-06-1 acrylamide 3 140-88-5 ethyl acrylate 4 - acrylic acid and its water-soluble salts 5 2439-35-2 2-(dimethylamino)ethyl acrylate 6 818-61-1 2-hydroxyethyl acrylate 7 141-32-2 n-butyl acrylate 8 96-33-3 methyl acrylate 9 107-13-1 acrylonitrile 10 107-02-8 acrolein 11 26628-22-8 sodium azide 12 75-07-0 acetaldehyde 13 75-05-8 acetonitrile 14 75-86-5 acetone cyanohydrin 15 83-32-9 acenaphthene 16 78-67-1 2,2'-azobisisobutyronitrile 17 90-04-0 o-anisidine 18 62-53-3 aniline 19 82-45-1 1-amino-9,10-anthraquinone 20 141-43-5 2-aminoethanol 21 1698-60-8 5-amino-4-chloro-2-phenylpyridazin-3(2H)-one; chloridazon 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-3-cyano- 22 120068-37-3 4[(trifluoromethyl)sulfinyl]pyrazole; fipronil 23 123-30-8 p-aminophenol 24 591-27-5 m-aminophenol 4-amino-6-tert-butyl-3-methylthio-1,2,4-triazin-5(4H)-one; 25 21087-64-9 metribuzin 26 107-11-9 3-amino-1-propene 27 41394-05-2 4-amino-3-methyl-6-phenyl-1,2,4-triazin-5(4H)-one; metamitron 28 107-18-6 allyl alcohol 29 106-92-3 1-allyloxy-2,3-epoxypropane 30 - n-alkylbenzenesulfonic acid and its salts(alkyl C=10-14) 31 - antimony and its compounds 32 120-12-7 anthracene 33 1332-21-4 asbestos ○ 34 4098-71-9 3-isocyanatomethyl-3,5,5-trimethylcyclohexyl isocyanate 35 78-84-2 isobutyraldehyde -

(12) Patent Application Publication (10) Pub. No.: US 2007/0059270 A1 Hall Et Al
US 2007005927OA1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0059270 A1 Hall et al. (43) Pub. Date: Mar. 15, 2007 (54) ION EXCHANGE RESIN TREATED TO (22) Filed: Sep. 13, 2005 CONTROL, SWELLING Publication Classification (75) Inventors: Harlan Hall, Oregon, WI (US); J. Scott Madsen, Cottage Grove, WI (US) (51) Int. Cl. A 6LX 3L/795 (2006.01) Correspondence Address: (52) U.S. Cl. ............................................................ 424/78.1 CHALKER FLORES, LLP 2711 LBJ FRWY (57) ABSTRACT Suite 1036 DALLAS, TX 75234 (US) The present invention provides a method and composition are provided that includes an ion exchange resin treated with (73) Assignee: The Coating Place, Inc., Verona, WI from between about 0.01 to about 10 percent by weight of one or more Sugar alcohols in contact with one or more ionic (21) Appl. No.: 11/225,834 pharmaceutically active drug. US 2007/0059270 A1 Mar. 15, 2007 ON EXCHANGE RESIN TREATED TO CONTROL drug bound to an ion-exchange resin to provide a drug-resin SWELLING complex having a drug content above a specified value. The drug-resin complex is Subsequently coated with a water TECHNICAL FIELD OF THE INVENTION permeable diffusion barrier coating that is insoluble in 0001. The present invention relates general to the con gastrointestinal fluids. Thus, the release of drug is controlled trolled release of active agents, and in particular, to phar under conditions encountered in the gastrointestinal tract. macologically active drugs adsorbed to ion exchange resin. 0007 One of the major disadvantages with the use of an ion exchange resin as a pharmaceutical delivery agent is that BACKGROUND OF THE INVENTION ion exchange resin particles are Susceptible to Swelling. -

Hazardous Waste Classification
HAZARDOUS WASTE CLASSIFICATION: REVIEW OF WORST CASE TO LESS WORST CASE METAL SPECIES WITH A WORKED EXAMPLE FOR A CONTAMINATED SOIL Ian Bishop 1,* and Pierre Hennebert 2 1 One Touch Data Ltd, Suite 4, Third Floor, Nicholsons House, Nicholsons Walk, Maidenhead SL6 1LD, United Kingdom 2 INERIS (National Institute for Industrial Environment and Risks), BP 2, F-60550 Verneuil-en-Halatte, France Article Info: ABSTRACT Received: The classification of waste as either hazardous or non-hazardous, especially for mix- 28 October 2020 Revised: tures such as contaminated soils, ashes, filter cakes and sludges, is not straight for- 17 February 2021 ward. In particular, as the laboratories can only measure total metal concentrations, Accepted: both the European and the UK technical guidance state that if the classifier doesn’t 25 February 2021 know exactly which metal species is in their waste, then they should start from a Available online: worst case species and use lines of evidence to work towards a more reasonable 31 March 2021 (less hazardous) species. However, the guidance doesn’t define or list worst case Keywords: nor less worst case species. While some authors have documented worst case spe- Hazardous properties cies, this is only in relation to documenting the concentrations at which each hazard Worst case Metal species property is triggered for a given worst case species. This paper addresses this gap. It documents how to define both the worst case species and more importantly, lists less worst case species for 32 elements and 204 metal species; species based on those listed in the European legislation but also supplemented by species that hav- en’t (yet) been included in this legislation but are significant nevertheless.