Hazardous Waste Classification
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Michigan Hop Management Guide 2018
2018 Michigan Hop Management Guide This material is based upon work supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, under Agreement No. 2015-09785. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture. 2 Table of Contents Growth Stages………………………………………………………………………………………3 Weed Management………………………………………………………………………….4-5 Herbicides………………..……………………………………………………………………….6-7 Fungicides……………………………..………………………………………………………….8-9 Insecticides…………………………..………………………………………………………10-11 Miticides…………………………………………………………………………………………….12 Pesticide Toxicity to Beneficial Insects…………………………………………..13-14 Nutrient Management Considerations…………………………………………15-19 Scouting Calendar………………………………………………………………………………20 Information presented here does not supersede the label directions. To protect yourself, others, and the environment, always read the label before applying any pesticide. Although efforts have been made to check the accuracy of information presented, it is the responsibility of the person using this information to verify that it is correct by reading the corresponding pesticide label in its entirety before using the product. The information presented here is intended as a guide for Michigan hop growers in selecting pesticides and is for educational purposes only. Labels can and do change. For current label and MSDS information, visit one of the following free online databases: greenbook.net, cdms.com, and agrian.com The efficacies of products listed have not been evaluated on hop in Michigan. Reference to commercial products or trade names does not imply endorsement by Michigan State University Extension or bias against those not mentioned. This information was compiled by Erin Lizotte and Dr. Robert Sirrine with assistance from Dr. -

Identifying the Cause of Sediment Toxicity in Agricultural Sediments: the Role of Pyrethroids and Nine Seldom-Measured Hydrophobic Pesticides ⇑ Donald P
Chemosphere 90 (2013) 958–964 Contents lists available at SciVerse ScienceDirect Chemosphere journal homepage: www.elsevier.com/locate/chemosphere Identifying the cause of sediment toxicity in agricultural sediments: The role of pyrethroids and nine seldom-measured hydrophobic pesticides ⇑ Donald P. Weston a, , Yuping Ding b, Minghua Zhang c, Michael J. Lydy b a Department of Integrative Biology, University of California, 1005 Valley Life Sciences Bldg., Berkeley, CA 94720-3140, USA b Fisheries and Illinois Aquaculture Center and Department of Zoology, Southern Illinois University, 171 Life Sciences II, Carbondale, IL 62901, USA c Department of Land, Air, and Water Resources, University of California, Davis, CA 95616, USA highlights " Monitoring fails to test for many agricultural pesticides used in any given area. " Nine seldom-analyzed pesticides (e.g., abamectin) were tested for in sediments. " One-quarter of the sediment samples were toxic to the amphipod, Hyalella azteca. " The seldom-analyzed pesticides may have contributed to toxicity in a few samples. " Pyrethroid insecticides were responsible for the vast majority of toxicity. article info abstract Article history: Few currently used agricultural pesticides are routinely monitored for in the environment. Even if Received 10 January 2012 concentrations are known, sediment LC50 values are often lacking for common sediment toxicity testing Received in revised form 16 May 2012 species. To help fill this data gap, sediments in California’s Central Valley were tested for nine hydropho- Accepted 27 June 2012 bic pesticides seldom analyzed: abamectin, diazinon, dicofol, fenpropathrin, indoxacarb, methyl para- Available online 23 July 2012 thion, oxyfluorfen, propargite, and pyraclostrobin. Most were detected, but rarely at concentrations acutely toxic to Hyalella azteca or Chironomus dilutus. -

The Calcium Arsenates
Station RuIletin 131. June, 1918 Oregon Agricultural College Experiment Station AGRICULTURAL CHEMISTRY DEPARTMENT The Calcium Arsenates By R. H. ROBINSON Acting Chemist, Oregon Agricultural Experiment Station. CORVALLIS, OREGON The regular huIlejne of the Station are sent free to the residents of Oregon who request them. THE CALCIUM ARSENATES By R. H. ROBINSON Acting Chemist, Oregon Agricultural Experiment Station INTRODUCTION Chemical investigations on the calcium arsenates relative to their economfic value and practicability as insecticides have been carried on by the department of Agricultural Chemistry of this Station during the past two years.The results obtained from these investigations are presented in this bulletin.The work was supported by the annual funds provided by the Adams Act of the United States Government.. Commercial calcium arsenate is an arsenical now being produced by reliable manufacturers of spray material and offered for sale as a sub- stitute for the arsenates of lead.The value of the latter as a stomachic insecticide has been demonstrated, and itis now used extensively for the successful controlof the codling moth, the destructionof the cotton boll worm., the tobacco worm, and the Colorado potato beetle. Previous inveatigations on the toxic values and killing power of calcium arsenate and lead arsenate indicate equal efficiency. A consideration of a few figures will show the economic advantages which might be gained if calcium arsenate could be substituted for lead arsenate.A conservative estimate of the quantity of lead arsenate used annually in the United States, as stated by one of the largest manufac- turers of spray materials, is probably more than 30,000,000 pounds. -

Biodegradation of Dicofol by Microbacterium Sp. D-2 Isolated
Lu et al. Appl Biol Chem (2019) 62:72 https://doi.org/10.1186/s13765-019-0480-y ARTICLE Open Access Biodegradation of dicofol by Microbacterium sp. D-2 isolated from pesticide-contaminated agricultural soil Peng Lu* , Hong‑ming Liu and Ai‑min Liu Abstract Dicofol is an organochlorine insecticide widely used to prevent pests worldwide. Consequently, serious environmen‑ tal problems have arisen from the application of dicofol. Bioremediation is an efective solution for dicofol persistence in the environment. In this study, a bacterial strain D‑2, identifed to genus Microbacterium, capable of degrading dicofol was isolated from dicofol‑contaminated agricultural soil. This represents the frst dicofol degrading bacterium isolated from this genus. Microbacterium sp. D‑2 degraded 50 mg/L dicofol within 24 h at a rate of 85.1%. Dicofol was dechlorinated by D‑2 and the further degradation metabolite was indentifed as p,p′‑dichlorobenzophenone(DCBP). Soils inoculated with Microbacterium sp. D‑2 degraded 81.9% of the dicofol, while soils without D‑2 only degraded 20.5% of the dicofol present. This fnding suggests that strain D‑2 has great potential in bioremediation of dicofol‑ contaminated soils. Keywords: Dicofol, Biodegradation, Microbacterium sp. D‑2, Bioremediation Introduction cause acute poising of fsh and shrimp, endocrine dis- Organochlorine pesticides (OCPs) are broad spectrum ruptive properties, and chronic toxicity to other organ- insecticides with high toxicity and long residual periods isms [10–13]. Terefore, it is believed that DCF would [1]. Tey have been widely used in the control of agricul- exert a negative infuence on both animals [14, 15] and tural pests, antisepsis in industrial production and the humans [16]. -

FENIX OUTDOOR Chemical Guideline and Restricted Substances
Management Guideline Number: SU-POL-FE-01-V04-2018-EN Version: 2.1 Pages: 1 of 82 Valid from: 2018-08-08 Chemical Guideline and Restricted Created by: var. au. /AB Substances List Approved by: Aiko Bode/Swerea FENIX OUTDOOR Chemical Guideline and Restricted Substances List (RSL) 1 Number: SU-POL-FE-01-V04-2018-EN Management Version: 2.1 Guideline Page: 2 of 82 Content 1. General Considerations ......................................................................................................... 4 2. Purpose ................................................................................................................................... 4 3. Scope of Application .............................................................................................................. 5 4. Additional Valid Instructions and Reference Documents ................................................... 5 5. Definition of Terms ................................................................................................................. 5 6. Duties and Responsibilities ................................................................................................... 7 7. Content – The Chemicals List ............................................................................................... 8 7.1 Process and Packaging-related Chemicals 8 7.1.1 Alkylphenol ethoxylates (APEO) and derivatives .......................................................................................... 8 7.1.2 Aliphatic organic solvents ............................................................................................................................. -

EWG VERIFIED™ Products Cannot Contain Any of the Ingredients Outlined in This Document
EWG’S UNACCEPTABLE LIST: Baby Diapers EWG VERIFIED™ products cannot contain any of the ingredients outlined in this document. Appendix A. Substances prohibited inEWG VERIFIED diapers based on GHS hazard classifications. A = aquatic toxicity, C = carcinogenicity, D = reproductive toxicity (development), F = reproductive toxicity (fertility), L = reproductive toxicity (lactation [breast-feeding children]), M = mutagenic, Sr = sensitization (respiratory), Ss =sensitization (skin) Chemical name(s) EC Number(s) CAS Number(s) Hazards ((4-phenylbutyl)hydroxyphosphoryl)acetic acid 412-170-7 83623-61-4 Ss (-)(3S,4R)-4-(4-fluorophenyl)-3-(3,4-methylenedioxy-phenoxymethyl)-N-benzylpiperidine hydrochloride 432-360-3 105813-13-6 SsA (+)-(1S,2S,3S,5R)-2,6,6-trimethylbicyclo[3.1.1]heptane-3-spiro-1'-(cyclohex-2'-en-4'-one) 430-460-1 133636-82-5 SsA (+/-)-(R*,R*)-6-fluoro-3,4-dihydro-2-oxiranyl-2H-1-benzopyran; 6-fluoro-2-(2-oxiranyl)chromane 419-620-1 - Ss (±) trans-3,3-dimethyl-5-(2,2,3-trimethyl-cyclopent-3-en-1-yl)-pent-4-en-2-ol 411-580-3 107898-54-4 A (±)-[(R*,R*) and (R*,S*)]-6-fluoro-3,4-dihydro-2-oxiranyl-2H-1-benzopyran 419-600-2 99199-90-3 Ss (±)-4-(3-chlorophenyl)-6-[(4-chlorophenyl)hydroxy(1-methyl-1H-imidazol-5-yl)methyl]-1-methyl-2(1H)-quinolin 430-730-9 - A (±)-4-[2-[[3-(4-hydroxyphenyl)-1-methylpropyl]amino]-1-hydroxyethyl]phenol hydrochloride 415-170-5 90274-24-1 Ss (±)-α-[(2-acetyl-5-methylphenyl)-amino]-2,6-dichlorobenzene-aceto-nitrile 419-290-9 Ss (1,3,4,5,6,7-hexahydro-1,3-di-oxo-2H-isoindol-2-yl)methyl (1R-trans)-2,2-dimethyl-3-(2-methylprop-1- -
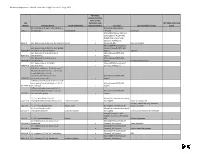
Chemicals of High Concern List (Sorted Alphabetically), July 2010
Minnesota Department of Health, Chemicals of High Concern list, July 1, 2010 Persistent, Bioaccumulative, Toxic or very CAS Persistent, very HPV (2006 and 3 of 4 Number Chemical Name Health endpoint(s) Bioaccumulative Source(s) Use example(s) or class years) (S)-4-hydroxy-3-(3-oxo-1-phenylbutyl)-2- Maine (EU Reproductive 5543-57-7 benzopyrone Reproduction Toxicant) Sunscreen Maine (CA Prop 65; IARC; EU Carcinogen; NTP 11th ROC; OSPAR Chemicals of High Concern); WA Appen1; 91-94-1 [1,1'-biphenyl]-4,4'-diamine, 3,3'-dichloro-Cancer x Minnesota HRL Dye, curing agent Maine (OSPAR Chemicals of [1,1'-biphenyl]-4,4'-diamine, N,N'-bis(2,4- Concern; Canada PBiT); WA 29398-96-7 dinitrophenyl)-3,3'-dimethoxy- x Appen1 Colorant [1,1'-Biphenyl]-4-ol, 3,4',5-tris(1,1- Maine (Canada PBiT); WA 6257-39-2 dimethylethyl)- x Appen1 [1,1'-Biphenyl]-4-ol, 3,4'-bis(1,1- Maine (Canada PBiT); WA 42479-88-9 dimethylethyl)- x Appen1 Chemical intermediate [1,1'-biphenyl]-4-ol, 3,5-bis(1,1- Maine (OSPAR Chemicals of 2668-47-5 dimethylethyl)- x Concern); WA Appen1 [2,6'-Bibenzothiazole]-7-sulfonic acid, 2'- (4-aminophenyl)-6-methyl-, diazotized, coupled with diazotized 4- aminobenzenesulfonic acid and Maine (Canada PBiT); WA 91696-90-1 resorcinol, sodium salts x Appen1 1(2H)-Quinolineethanol, 6-[(2-chloro-4,6- dinitrophenyl) azo]-3,4-dihydro-2,2,4,7- Maine (Canada PBiT); WA 63133-84-6 tetramethyl- x Appen1 1(2H)-Quinolinepropanamide, 6-(2,2- dicyanoethenyl)-3, 4-dihydro-2,2,4,7- Maine (Canada PBiT); WA 63467-15-2 tetramethyl-N-phenyl- x Appen1 1,1,1,2-Tetrachloro-2,2-bis(4- -

2019 Minnesota Chemicals of High Concern List
Minnesota Department of Health, Chemicals of High Concern List, 2019 Persistent, Bioaccumulative, Toxic (PBT) or very Persistent, very High Production CAS Bioaccumulative Use Example(s) and/or Volume (HPV) Number Chemical Name Health Endpoint(s) (vPvB) Source(s) Chemical Class Chemical1 Maine (CA Prop 65; IARC; IRIS; NTP Wood and textiles finishes, Cancer, Respiratory 11th ROC); WA Appen1; WA CHCC; disinfection, tissue 50-00-0 Formaldehyde x system, Eye irritant Minnesota HRV; Minnesota RAA preservative Gastrointestinal Minnesota HRL Contaminant 50-00-0 Formaldehyde (in water) system EU Category 1 Endocrine disruptor pesticide 50-29-3 DDT, technical, p,p'DDT Endocrine system Maine (CA Prop 65; IARC; IRIS; NTP PAH (chem-class) 11th ROC; OSPAR Chemicals of Concern; EuC Endocrine Disruptor Cancer, Endocrine Priority List; EPA Final PBT Rule for 50-32-8 Benzo(a)pyrene x x system TRI; EPA Priority PBT); Oregon P3 List; WA Appen1; Minnesota HRV WA Appen1; Minnesota HRL Dyes and diaminophenol mfg, wood preservation, 51-28-5 2,4-Dinitrophenol Eyes pesticide, pharmaceutical Maine (CA Prop 65; IARC; NTP 11th Preparation of amino resins, 51-79-6 Urethane (Ethyl carbamate) Cancer, Development ROC); WA Appen1 solubilizer, chemical intermediate Maine (CA Prop 65; IARC; IRIS; NTP Research; PAH (chem-class) 11th ROC; EPA Final PBT Rule for 53-70-3 Dibenzo(a,h)anthracene Cancer x TRI; WA PBT List; OSPAR Chemicals of Concern); WA Appen1; Oregon P3 List Maine (CA Prop 65; NTP 11th ROC); Research 53-96-3 2-Acetylaminofluorene Cancer WA Appen1 Maine (CA Prop 65; IARC; IRIS; NTP Lubricant, antioxidant, 55-18-5 N-Nitrosodiethylamine Cancer 11th ROC); WA Appen1 plastics stabilizer Maine (CA Prop 65; IRIS; NTP 11th Pesticide (EPA reg. -

Addressing Highly Hazardous Pesticides in Mozambique
República de Moçambique Ministério da Agricultura e Segurança Alimentar Direcção Nacional de Agricultura e Silvicultura Addressing Highly Hazardous Pesticides in Mozambique Addressing Highly Hazardous Pesticides in Mozambique Food and Agriculture Organization of the United Nations Rome, 2016 The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. The views expressed in this information product are those of the author(s) and do not necessarily reflect the views or policies of FAO. ISBN 978-92-5-108519-6 © FAO, 2016 FAO encourages the use, reproduction and dissemination of material in this information product. Except where otherwise indicated, material may be copied, downloaded and printed for private study, research and teaching purposes, or for use in non-commercial products or services, provided that appropriate acknowledgement of FAO as the source and copyright holder is given and that FAO’s endorsement of users’ views, products or services is not implied in any way. All requests for translation and adaptation rights, and for resale and other commercial use rights should be made via www.fao.org/contact-us/licence-request or addressed to [email protected]. -

THE CHEMISTRY of PESTICIDES Walter R
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Food and Drug Administration Papers U.S. Department of Health and Human Services 6-23-1969 THE CHEMISTRY OF PESTICIDES Walter R. Benson Food and Drug Administration Follow this and additional works at: http://digitalcommons.unl.edu/usfda Part of the Dietetics and Clinical Nutrition Commons, Health and Medical Administration Commons, Health Services Administration Commons, Pharmaceutical Preparations Commons, and the Pharmacy Administration, Policy and Regulation Commons Benson, Walter R., "THE CHEMISTRY OF PESTICIDES" (1969). Food and Drug Administration Papers. 12. http://digitalcommons.unl.edu/usfda/12 This Article is brought to you for free and open access by the U.S. Department of Health and Human Services at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Food and Drug Administration Papers by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Reproduced by the U. S. DEPARTME",T OF HEAL TI!, EDUCATION, AND WELFARE Fc·od and Drug Admilri.stration THE CHEMISTRY OF PESTICIDES Walter R. Benson Pesticide Branch, Division of Food Chemistry, Bureau of Science Food and Drug Administration, Washington, D. C. Reprinted from ANNALS OF THE NEW YORK ACADEMY OF SCIENCES Volume 160, Article 1, Pages 7-29 June 23, 1969 THE CHEMISTRY OF PESTICIDES WaIter R. Benson Pesticide.f Brallch, DivisiO/l of Food Chemistr}" Bureall of Science "-ood and Drug Administration! Washingtoll, D. C. INTRODUCTION This review is limited to the structures ana a few reactions of the pesticides mainly in~ecticides-that affect mammalian systems and that are the subject of papers by other authors in this monograph. -

Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 Theinternational Programme on Chemical Safety (IPCS) Was Established in 1980
The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 cation Hazard of Pesticides by and Guidelines to Classi The WHO Recommended Classi The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 TheInternational Programme on Chemical Safety (IPCS) was established in 1980. The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. This publication was developed in the IOMC context. The contents do not necessarily reflect the views or stated policies of individual IOMC Participating Organizations. The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase international coordination in the field of chemical safety. The Participating Organizations are: FAO, ILO, UNDP, UNEP, UNIDO, UNITAR, WHO, World Bank and OECD. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment. WHO recommended classification of pesticides by hazard and guidelines to classification, 2019 edition ISBN 978-92-4-000566-2 (electronic version) ISBN 978-92-4-000567-9 (print version) ISSN 1684-1042 © World Health Organization 2020 Some rights reserved. -

Pesticide Residues : Maximum Residue Limits
THAI AGRICULTURAL STANDARD TAS 9002-2013 PESTICIDE RESIDUES : MAXIMUM RESIDUE LIMITS National Bureau of Agricultural Commodity and Food Standards Ministry of Agriculture and Cooperatives ICS 67.040 ISBN UNOFFICAL TRANSLATION THAI AGRICULTURAL STANDARD TAS 9002-2013 PESTICIDE RESIDUES : MAXIMUM RESIDUE LIMITS National Bureau of Agricultural Commodity and Food Standards Ministry of Agriculture and Cooperatives 50 Phaholyothin Road, Ladyao, Chatuchak, Bangkok 10900 Telephone (662) 561 2277 Fascimile: (662) 561 3357 www.acfs.go.th Published in the Royal Gazette, Announcement and General Publication Volume 131, Special Section 32ง (Ngo), Dated 13 February B.E. 2557 (2014) (2) Technical Committee on the Elaboration of the Thai Agricultural Standard on Maximum Residue Limits for Pesticide 1. Mrs. Manthana Milne Chairperson Department of Agriculture 2. Mrs. Thanida Harintharanon Member Department of Livestock Development 3. Mrs. Kanokporn Atisook Member Department of Medical Sciences, Ministry of Public Health 4. Mrs. Chuensuke Methakulawat Member Office of the Consumer Protection Board, The Prime Minister’s Office 5. Ms. Warunee Sensupa Member Food and Drug Administration, Ministry of Public Health 6. Mr. Thammanoon Kaewkhongkha Member Office of Agricultural Regulation, Department of Agriculture 7. Mr. Pisan Pongsapitch Member National Bureau of Agricultural Commodity and Food Standards 8. Ms. Wipa Thangnipon Member Office of Agricultural Production Science Research and Development, Department of Agriculture 9. Ms. Pojjanee Paniangvait Member Board of Trade of Thailand 10. Mr. Charoen Kaowsuksai Member Food Processing Industry Club, Federation of Thai Industries 11. Ms. Natchaya Chumsawat Member Thai Agro Business Association 12. Mr. Sinchai Swasdichai Member Thai Crop Protection Association 13. Mrs. Nuansri Tayaputch Member Expert on Method of Analysis 14.