The Calcium Arsenates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FENIX OUTDOOR Chemical Guideline and Restricted Substances
Management Guideline Number: SU-POL-FE-01-V04-2018-EN Version: 2.1 Pages: 1 of 82 Valid from: 2018-08-08 Chemical Guideline and Restricted Created by: var. au. /AB Substances List Approved by: Aiko Bode/Swerea FENIX OUTDOOR Chemical Guideline and Restricted Substances List (RSL) 1 Number: SU-POL-FE-01-V04-2018-EN Management Version: 2.1 Guideline Page: 2 of 82 Content 1. General Considerations ......................................................................................................... 4 2. Purpose ................................................................................................................................... 4 3. Scope of Application .............................................................................................................. 5 4. Additional Valid Instructions and Reference Documents ................................................... 5 5. Definition of Terms ................................................................................................................. 5 6. Duties and Responsibilities ................................................................................................... 7 7. Content – The Chemicals List ............................................................................................... 8 7.1 Process and Packaging-related Chemicals 8 7.1.1 Alkylphenol ethoxylates (APEO) and derivatives .......................................................................................... 8 7.1.2 Aliphatic organic solvents ............................................................................................................................. -

EWG VERIFIED™ Products Cannot Contain Any of the Ingredients Outlined in This Document
EWG’S UNACCEPTABLE LIST: Baby Diapers EWG VERIFIED™ products cannot contain any of the ingredients outlined in this document. Appendix A. Substances prohibited inEWG VERIFIED diapers based on GHS hazard classifications. A = aquatic toxicity, C = carcinogenicity, D = reproductive toxicity (development), F = reproductive toxicity (fertility), L = reproductive toxicity (lactation [breast-feeding children]), M = mutagenic, Sr = sensitization (respiratory), Ss =sensitization (skin) Chemical name(s) EC Number(s) CAS Number(s) Hazards ((4-phenylbutyl)hydroxyphosphoryl)acetic acid 412-170-7 83623-61-4 Ss (-)(3S,4R)-4-(4-fluorophenyl)-3-(3,4-methylenedioxy-phenoxymethyl)-N-benzylpiperidine hydrochloride 432-360-3 105813-13-6 SsA (+)-(1S,2S,3S,5R)-2,6,6-trimethylbicyclo[3.1.1]heptane-3-spiro-1'-(cyclohex-2'-en-4'-one) 430-460-1 133636-82-5 SsA (+/-)-(R*,R*)-6-fluoro-3,4-dihydro-2-oxiranyl-2H-1-benzopyran; 6-fluoro-2-(2-oxiranyl)chromane 419-620-1 - Ss (±) trans-3,3-dimethyl-5-(2,2,3-trimethyl-cyclopent-3-en-1-yl)-pent-4-en-2-ol 411-580-3 107898-54-4 A (±)-[(R*,R*) and (R*,S*)]-6-fluoro-3,4-dihydro-2-oxiranyl-2H-1-benzopyran 419-600-2 99199-90-3 Ss (±)-4-(3-chlorophenyl)-6-[(4-chlorophenyl)hydroxy(1-methyl-1H-imidazol-5-yl)methyl]-1-methyl-2(1H)-quinolin 430-730-9 - A (±)-4-[2-[[3-(4-hydroxyphenyl)-1-methylpropyl]amino]-1-hydroxyethyl]phenol hydrochloride 415-170-5 90274-24-1 Ss (±)-α-[(2-acetyl-5-methylphenyl)-amino]-2,6-dichlorobenzene-aceto-nitrile 419-290-9 Ss (1,3,4,5,6,7-hexahydro-1,3-di-oxo-2H-isoindol-2-yl)methyl (1R-trans)-2,2-dimethyl-3-(2-methylprop-1- -

The Insecticide Industry of Today Seed Production in Various States Has Comprises More Than 50 Basic Producers Doubled the Yield
put nearly 2 million dollars extra in the growers' pockets. In Mississippi at least 75 percent of the 1950 cotton crop The Insecticide would have been destroyed were it not for the control of insects through the Industry use of the industry's products. Insecti- cides applied in Nebraska to control Lea S. Hitchner grasshoppers in 1949 resulted in savings estimated at 2 million dollars. Insecti- cidal treatment of alfalfa raised for The insecticide industry of today seed production in various States has comprises more than 50 basic producers doubled the yield. or manufacturers and more than 500 One factor among others responsible formulatorsj xemixers, and processors. for the high productivity of American From their plants throughout the coun- agriculture is the cooperative attack try comes a great variety of insecticides that is waged on insects and other pests. and related products. The agricultural chemicals industry The products, except those derived has welcomed the opportunity to co- from botanical sources, have their ori- operate with Federal and State agen- gins in the basic chemicals on which cies and with farm organizations in this the industry is founded, but the proc- important work and to carry the re- esses that turn the raw materials into sponsibility for developing, producing, the finished products applied by farm- and delivering the necessary pesticides. ers are long, highly scientific, and ex- Such a responsibility is a heavy one pensive in capital. investment and even in normal times. It becomes operating costs. acutely heavy in times of national The industry employs thousands of stress, when shortages of raw materials, scientists in the fields of entomology, containers, personnel, and transporta- plant pathology, botany, toxicology, tion may hamper production and dis- medicine, chemistry, and chemical en- tribution. -

Chromium, Copper and Arsenic Oxide)-Treated Wood Hata, T.; Bronsveld, Paulus; Vystavel, T.; Kooi, Bart; De Hosson, J.T.M.; Kakitani, T.; Otono, A.; Imamura, Y
University of Groningen Electron microscopic study on pyrolysis of CCA (chromium, copper and arsenic oxide)-treated wood Hata, T.; Bronsveld, Paulus; Vystavel, T.; Kooi, Bart; De Hosson, J.T.M.; Kakitani, T.; Otono, A.; Imamura, Y. Published in: Journal of Analytical and Applied Pyrolysis DOI: 10.1016/S0165-2370(03)00077-9 IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2003 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Hata, T., Bronsveld, P. M., Vystavel, T., Kooi, B. J., de Hosson, J. T. M., Kakitani, T., ... Imamura, Y. (2003). Electron microscopic study on pyrolysis of CCA (chromium, copper and arsenic oxide)-treated wood. Journal of Analytical and Applied Pyrolysis, 68-9(1), 635-643. DOI: 10.1016/S0165-2370(03)00077-9 Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum. -

Table 2. Acute Dose-Response Values for Screening Risk Assessments
Date: 6/18/2018 Revisions since 9/18/2014 are shown in red. Table 2. Acute Dose-Response Values for Screening Risk Assessments. MRL = ATSDR minumum risk levels for no adverse effects for 1 to 14-day exposures. REL = California EPA reference exposure level for no adverse effects. Most, but not all, RELs are for 1-hour exposures. AEGL = Acute exposure guideline levels for mild effects (AEGL-1) and moderate effects (AEGL-2) for 1- and 8-hour exposures. Superscripts indicate the AEGL's status: f = final, i=interim, and p=proposed. ERPG = US DOE Emergency Removal Program guidelines for mild or transient effects (ERPG-1) and irreversible or serious effects (ERPG-2) for 1-hour exposures. IDLH/10 = One-tenth of levels determined by NIOSH to be imminently dangerous to life and health, approximately comparable to mild effects levels for 1-hour exposures. IDLH/10 values shown here are only for substances that lack AEGL and ERPG values. TEEL = US DOE Temporary emergency exposure limits for mild, transient effects (TEEL-1) for 1-hour exposures. TEELs are derived according to a tiered, formula-like methodology, and do not undergo peer review. They are not recommended as the basis for regulatory desision-making, and are shown here only to inform situations where acute values from other sources are not available. Table 2. Acute Dose-Response Values for Screening Risk Assessments MRL REL AEGL-1 (1-h) AEGL-1 (8-h) AEGL-2 (1-h) AEGL-2 (8-h) ERPG-1 ERPG-2 IDLH/10 TEEL-1 CHEMICAL NAME CAS NO. -

Chemical List
1 EXHIBIT 1 2 CHEMICAL CLASSIFICATION LIST 3 4 1. Pyrophoric Chemicals 5 1.1. Aluminum alkyls: R3Al, R2AlCl, RAlCl2 6 Examples: Et3Al, Et2AlCl, EtAlCl2, Me3Al, Diethylethoxyaluminium 7 1.2. Grignard Reagents: RMgX (R=alkyl, aryl, vinyl X=halogen) 8 1.3. Lithium Reagents: RLi (R = alkyls, aryls, vinyls) 9 Examples: Butyllithium, Isobutyllithium, sec-Butyllithium, tert-Butyllithium, 10 Ethyllithium, Isopropyllithium, Methyllithium, (Trimethylsilyl)methyllithium, 11 Phenyllithium, 2-Thienyllithium, Vinyllithium, Lithium acetylide ethylenediamine 12 complex, Lithium (trimethylsilyl)acetylide, Lithium phenylacetylide 13 1.4. Zinc Alkyl Reagents: RZnX, R2Zn 14 Examples: Et2Zn 15 1.5. Metal carbonyls: Lithium carbonyl, Nickel tetracarbonyl, Dicobalt octacarbonyl 16 1.6. Metal powders (finely divided): Bismuth, Calcium, Cobalt, Hafnium, Iron, 17 Magnesium, Titanium, Uranium, Zinc, Zirconium 18 1.7. Low Valent Metals: Titanium dichloride 19 1.8. Metal hydrides: Potassium Hydride, Sodium hydride, Lithium Aluminum Hydride, 20 Diethylaluminium hydride, Diisobutylaluminum hydride 21 1.9. Nonmetal hydrides: Arsine, Boranes, Diethylarsine, diethylphosphine, Germane, 22 Phosphine, phenylphosphine, Silane, Methanetellurol (CH3TeH) 23 1.10. Non-metal alkyls: R3B, R3P, R3As; Tributylphosphine, Dichloro(methyl)silane 24 1.11. Used hydrogenation catalysts: Raney nickel, Palladium, Platinum 25 1.12. Activated Copper fuel cell catalysts, e.g. Cu/ZnO/Al2O3 26 1.13. Finely Divided Sulfides: Iron Sulfides (FeS, FeS2, Fe3S4), and Potassium Sulfide 27 (K2S) 28 REFERRAL -

Inorganic Arsenic Compounds Other Than Arsine Health and Safety Guide
OS INTERNATiONAL I'ROGRAMME ON CHEMICAL SAFETY Health and Safety Guide No. 70 INORGANIC ARSENIC COMPOUNDS OTHER THAN ARSINE HEALTH AND SAFETY GUIDE i - I 04 R. Q) UNEP UNITED NATIONS INTERNATIONAL ENVIRONMENT I'R( )GRAMME LABOUR ORGANISATION k\s' I V WORLD HEALTH ORGANIZATION WORLD HEALTH ORGANIZATION, GENEVA 1992 IPcs Other H EA LTH AND SAFETY GUIDES available: Aerytonitrile 41. Clii rdeon 2. Kekvau 42. Vatiadiuni 3 . I Bula not 43 Di meLhyI ftirmatnide 4 2-Buta101 44 1-Dryliniot 5. 2.4- Diehlorpheiioxv- 45 . Ac rylzi mule acetic Acid (2.4-D) 46. Barium 6. NIcihylene Chhride 47. Airaziiie 7 . ie,i-Buia nol 48. Benlm'.ie 8. Ep Ichioroli) Olin 49. Cap a 64 P. ls.ihutaiiol 50. Captaii I o. feiddin oeth N lene Si. Parai.tuat II. Tetradi ion 51 Diquat 12. Te nacelle 53. Alpha- and Betal-lexachloro- 13 Clils,i (lane cyclohexanes 14 1 kpia Idor 54. Liiidaiic IS. Propylene oxide 55. 1 .2-Diciilroetiiane Ethylene Oxide 5t. Hydrazine Eiulosiillaii 57. F-orivaldehydc IS. Die h lorvos 55. MLhyI Isobu I V I kcloiic IV. Pculaehloro1heiiol 59. fl-Flexaric 20. Diiiiethoaie 61), Endrin 2 1 . A iii in and Dick) 0in 6 I . I sh IIZiLI1 22. Cyperniellirin 62. Nicki. Nickel Caution I. and some 23. Quiiiloieiic Nickel Compounds 24. Alkthrins 03. Hexachlorocyclopeuladiene 25. Rsiiiethii ins 64. Aidicaib 26. Pyr rot ii,id inc Alkaloids 65. Fe nitrolhioit 27. Magnetic Fields hib. Triclilorlon 28. Phosphine 67. Acroleiii 29. Diiiiethyl Sull'ite 68. Polychlurinated hiphenyls (PCBs) and 30. Dc lianteth nil polyc h In ruiated letlilienyls (fs) 31. -
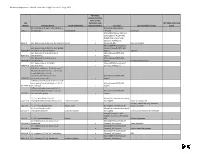
Chemicals of High Concern List (Sorted Alphabetically), July 2010
Minnesota Department of Health, Chemicals of High Concern list, July 1, 2010 Persistent, Bioaccumulative, Toxic or very CAS Persistent, very HPV (2006 and 3 of 4 Number Chemical Name Health endpoint(s) Bioaccumulative Source(s) Use example(s) or class years) (S)-4-hydroxy-3-(3-oxo-1-phenylbutyl)-2- Maine (EU Reproductive 5543-57-7 benzopyrone Reproduction Toxicant) Sunscreen Maine (CA Prop 65; IARC; EU Carcinogen; NTP 11th ROC; OSPAR Chemicals of High Concern); WA Appen1; 91-94-1 [1,1'-biphenyl]-4,4'-diamine, 3,3'-dichloro-Cancer x Minnesota HRL Dye, curing agent Maine (OSPAR Chemicals of [1,1'-biphenyl]-4,4'-diamine, N,N'-bis(2,4- Concern; Canada PBiT); WA 29398-96-7 dinitrophenyl)-3,3'-dimethoxy- x Appen1 Colorant [1,1'-Biphenyl]-4-ol, 3,4',5-tris(1,1- Maine (Canada PBiT); WA 6257-39-2 dimethylethyl)- x Appen1 [1,1'-Biphenyl]-4-ol, 3,4'-bis(1,1- Maine (Canada PBiT); WA 42479-88-9 dimethylethyl)- x Appen1 Chemical intermediate [1,1'-biphenyl]-4-ol, 3,5-bis(1,1- Maine (OSPAR Chemicals of 2668-47-5 dimethylethyl)- x Concern); WA Appen1 [2,6'-Bibenzothiazole]-7-sulfonic acid, 2'- (4-aminophenyl)-6-methyl-, diazotized, coupled with diazotized 4- aminobenzenesulfonic acid and Maine (Canada PBiT); WA 91696-90-1 resorcinol, sodium salts x Appen1 1(2H)-Quinolineethanol, 6-[(2-chloro-4,6- dinitrophenyl) azo]-3,4-dihydro-2,2,4,7- Maine (Canada PBiT); WA 63133-84-6 tetramethyl- x Appen1 1(2H)-Quinolinepropanamide, 6-(2,2- dicyanoethenyl)-3, 4-dihydro-2,2,4,7- Maine (Canada PBiT); WA 63467-15-2 tetramethyl-N-phenyl- x Appen1 1,1,1,2-Tetrachloro-2,2-bis(4- -

Code Chemical P026 1-(O-Chlorophenyl)Thiourea P081 1
Code Chemical P026 1-(o-Chlorophenyl)thiourea P081 1,2,3-Propanetriol, trinitrate (R) P042 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, (R)- P067 1,2-Propylenimine P185 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)- carbonyl]oxime 1,4,5,8-Dimethanonaphthalene, 1,2,3,4,10,10-hexa- chloro-1,4,4a,5,8,8a,-hexahydro-, P004 (1alpha,4alpha, 4abeta,5alpha,8alpha,8abeta)- 1,4,5,8-Dimethanonaphthalene, 1,2,3,4,10,10-hexa- chloro-1,4,4a,5,8,8a-hexahydro-, P060 (1alpha,4alpha, 4abeta,5beta,8beta,8abeta)- P002 1-Acetyl-2-thiourea P048 2,4-Dinitrophenol P051 2,7:3,6-Dimethanonaphth [2,3-b]oxirene, 3,4,5,6,9,9 -hexachloro-1a,2,2a,3,6,6a,7,7a- octahydro-, (1aalpha,2beta,2abeta,3alpha,6alpha,6abeta,7 beta, 7aalpha)-, & metabolites 2,7:3,6-Dimethanonaphth[2,3-b]oxirene, 3,4,5,6,9,9- hexachloro-1a,2,2a,3,6,6a,7,7a- P037 octahydro-, (1aalpha,2beta,2aalpha,3beta,6beta,6aalpha,7 beta, 7aalpha)- P045 2-Butanone, 3,3-dimethyl-1-(methylthio)-, O-[methylamino)carbonyl] oxime P034 2-Cyclohexyl-4,6-dinitrophenol 2H-1-Benzopyran-2-one, 4-hydroxy-3-(3-oxo-1- phenylbutyl)-, & salts, when present at P001 concentrations greater than 0.3% P069 2-Methyllactonitrile P017 2-Propanone, 1-bromo- P005 2-Propen-1-ol P003 2-Propenal P102 2-Propyn-1-ol P007 3(2H)-Isoxazolone, 5-(aminomethyl)- P027 3-Chloropropionitrile P047 4,6-Dinitro-o-cresol, & salts P059 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro- 3a,4,7,7a-tetrahydro- P008 4-Aminopyridine P008 4-Pyridinamine P007 5-(Aminomethyl)-3-isoxazolol 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- -

2019 Minnesota Chemicals of High Concern List
Minnesota Department of Health, Chemicals of High Concern List, 2019 Persistent, Bioaccumulative, Toxic (PBT) or very Persistent, very High Production CAS Bioaccumulative Use Example(s) and/or Volume (HPV) Number Chemical Name Health Endpoint(s) (vPvB) Source(s) Chemical Class Chemical1 Maine (CA Prop 65; IARC; IRIS; NTP Wood and textiles finishes, Cancer, Respiratory 11th ROC); WA Appen1; WA CHCC; disinfection, tissue 50-00-0 Formaldehyde x system, Eye irritant Minnesota HRV; Minnesota RAA preservative Gastrointestinal Minnesota HRL Contaminant 50-00-0 Formaldehyde (in water) system EU Category 1 Endocrine disruptor pesticide 50-29-3 DDT, technical, p,p'DDT Endocrine system Maine (CA Prop 65; IARC; IRIS; NTP PAH (chem-class) 11th ROC; OSPAR Chemicals of Concern; EuC Endocrine Disruptor Cancer, Endocrine Priority List; EPA Final PBT Rule for 50-32-8 Benzo(a)pyrene x x system TRI; EPA Priority PBT); Oregon P3 List; WA Appen1; Minnesota HRV WA Appen1; Minnesota HRL Dyes and diaminophenol mfg, wood preservation, 51-28-5 2,4-Dinitrophenol Eyes pesticide, pharmaceutical Maine (CA Prop 65; IARC; NTP 11th Preparation of amino resins, 51-79-6 Urethane (Ethyl carbamate) Cancer, Development ROC); WA Appen1 solubilizer, chemical intermediate Maine (CA Prop 65; IARC; IRIS; NTP Research; PAH (chem-class) 11th ROC; EPA Final PBT Rule for 53-70-3 Dibenzo(a,h)anthracene Cancer x TRI; WA PBT List; OSPAR Chemicals of Concern); WA Appen1; Oregon P3 List Maine (CA Prop 65; NTP 11th ROC); Research 53-96-3 2-Acetylaminofluorene Cancer WA Appen1 Maine (CA Prop 65; IARC; IRIS; NTP Lubricant, antioxidant, 55-18-5 N-Nitrosodiethylamine Cancer 11th ROC); WA Appen1 plastics stabilizer Maine (CA Prop 65; IRIS; NTP 11th Pesticide (EPA reg. -

Florida Pesticide Reporting Guidelines
Florida Pesticide Reporting guidelines This list is compiled from the Environmental Protection Agency List of Lists (2015) and updated with common Pesticide Trade Names. This is meant as a supplement to the EPA list of lists to clarify and assist handlers and responders in the field to Florida reporting requirements and the more common chemical nomenclature. Threshold Planning Quantity (TPQ) – The presence of Extremely Hazardous Substances (EHSs) in quantities at or above the Threshold Planning Quantity (TPQ) requires certain emergency planning activities to be conducted. The consolidated list presents the TPQ (in pounds) for section 302 chemicals in the column following the CAS number. For chemicals that are solids, there are two TPQs given (e.g., 500/10,000). In these cases, the lower quantity applies for solids in powder form with particle size less than 100 microns, or if the substance is in solution or in molten form. Otherwise, the 10,000 pound TPQ applies. Section 304 RQ‐ Facilities must immediately report accidental releases of EHS chemicals and "hazardous substances" in quantities greater than corresponding Reportable Quantities (RQs) defined under the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) to state and local officials. Information about accidental chemical releases must be available to the public, Florida Reporting requirements below. CERCLA RQ‐ Releases of CERCLA hazardous substances, in quantities equal to or greater than their reportable quantity (RQ) in pounds, are subject to reporting to the Florida Reporting requirements below. Florida Reporting requirements: National Response Center Florida State Watch Office (800) 424-8802 (800) 320-0519 or (850) 815-4001 Florida Department of Environmental Protection Spill reporting requirements https://floridadep.gov/pollutionnotice Florida Division of Emergency Management 2555 Shumard Oak blvd. -

Hazardous Waste Classification
HAZARDOUS WASTE CLASSIFICATION: REVIEW OF WORST CASE TO LESS WORST CASE METAL SPECIES WITH A WORKED EXAMPLE FOR A CONTAMINATED SOIL Ian Bishop 1,* and Pierre Hennebert 2 1 One Touch Data Ltd, Suite 4, Third Floor, Nicholsons House, Nicholsons Walk, Maidenhead SL6 1LD, United Kingdom 2 INERIS (National Institute for Industrial Environment and Risks), BP 2, F-60550 Verneuil-en-Halatte, France Article Info: ABSTRACT Received: The classification of waste as either hazardous or non-hazardous, especially for mix- 28 October 2020 Revised: tures such as contaminated soils, ashes, filter cakes and sludges, is not straight for- 17 February 2021 ward. In particular, as the laboratories can only measure total metal concentrations, Accepted: both the European and the UK technical guidance state that if the classifier doesn’t 25 February 2021 know exactly which metal species is in their waste, then they should start from a Available online: worst case species and use lines of evidence to work towards a more reasonable 31 March 2021 (less hazardous) species. However, the guidance doesn’t define or list worst case Keywords: nor less worst case species. While some authors have documented worst case spe- Hazardous properties cies, this is only in relation to documenting the concentrations at which each hazard Worst case Metal species property is triggered for a given worst case species. This paper addresses this gap. It documents how to define both the worst case species and more importantly, lists less worst case species for 32 elements and 204 metal species; species based on those listed in the European legislation but also supplemented by species that hav- en’t (yet) been included in this legislation but are significant nevertheless.