Summary of Product Characteristics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Safety Data Sheet TSP - TRISODIUM PHOSPHATE
Safety Data Sheet TSP - TRISODIUM PHOSPHATE Date of Revision: 2/11/2015 Section 1 – Chemical Product and Company Identification Product/Chemical Name: Trisodium Phosphate dodecahydrate Chemical Formula: Na3PO4*12H2O CAS Number: 10101-89-0 Other Designations: TSP; trisodium orthophosphate; tribasic; tertiary sodium phosphate; trisodium phosphate Derivation: Prepared by combining proper proportions of phosphoric acid and soda to form disodium phosphate, then adding a caustic soda Supplied by: PRO Chemical & Dye 126 Shove Street Fall River, MA 02724 Emergency Telephone Numbers: 800-255-3924 ChemTel. (United States) + 1 01 813-248-0585 (Outside the United States) 1. Section 2 - Hazards Identification HMIS ***** Emergency Overview ***** H 3 MAY CAUSE EYE INJURY. CAUSES SKIN IRRITATlON. MAYBE HARMFUL IF SWALLOWED. F 0 Potential Health Effects R 0 PPE Primary Entry Routes: Inhalation, ingestion or skin contact. Sec. 8 Target Organs: Skin, digestive tract. HAZARDS IDENTIFICATION Classification of the substance or mixture GHS Classification in accordance with 29 CFR 1910 (OSHA DCS) Skin corrosion (Category I B). H314 Serious eye damage (Category I). H318 GHS Label elements, including precautionary statements Pictogram Signal word Danger Hazard statement(s) H314 Causes severe skin burns and eye damage. Precautionary Statement(s) P260 Do not breathe dust or mist. P264 Wash skin thoroughly after handling. P280 Wear protective gloves protective clothing/ eye protection/ face protection. P301 + P330 + P331 IF SWALLOWED: rinse mouth. DO NOT induce vomiting. P303 + P36l + P353 IF ON SKIN (or hair): Remove. Take off immediately all contaminated clothing. Rinse skin with water / shower. P304 + P340 IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing. -

Brochure-Product-Range.Pdf
PRODUCT RANGE 2015 edition ANSI Standard 60 NSF® CERTIFIED HALAL M ISLAMIC FOOD AND NUTRITION ® COUNCIL OF AMERICA Rue Joseph Wauters, 144 ISO 9001:2008 (Quality) / OHSAS 18001:2007 (Health/ B-4480 Engis Safety) / ISO 14001:2004 (Environment) / ISO 22000:2005 www.globulebleu.com (Food Safety) / FSSC 22000:2013 (Food Safety). Tel. +32 (0) 4 273 93 58 Our food grade phosphates are allergen free, GMO free, Fax. +32 (0) 4 275 68 36 BSE/TSE free. www.prayon.com mail. [email protected] Design by www.prayon.com PRODUCT RANGE | 11 TABLE OF CONTENTS HORTICULTURE APPLICATIONS HORTIPRAY® RANGE FOR HORTICULTURE* FOOD AND INDUSTRIAL APPLICATIONS PRODUCT NAME Bulk density P O pH N-NH Made 2 5 4 MONOAMMONIUM PHOSPHATE - NH4H2PO4 in 3 3 % 1% % Sodium orthophosphates ................................................................................... 03 g/cm lbs/ft indicative indicative indicative Water-soluble fertilisers. Sodium pyrophosphates .................................................................................... 04 HORTIPRAY® MAP Horticultural Grade 0.9 56 61 4.5 12 Sodium tripolyphosphates ................................................................................. 05 HORTIPRAY® MAP 12.60 Horticultural Grade 0.9 56 60 5 12.1 Water-soluble fertilisers; Sodium polyphosphates ..................................................................................... 06 HORTIPRAY® MAP anticalc Horticultural Grade 0.9 56 61 4.5 12 preventive action against clogging. Potassium orthophosphates ............................................................................. -

Vaccine Excipient Table
Vaccine Excipient Summary Excipients Included in U.S. Vaccines, by Vaccine In addition to weakened or killed disease antigens (viruses or bacteria), vaccines contain very small amounts of other ingredients – excipients. Some excipients are added to a vaccine for a specific purpose. These include: Preservatives, to prevent contamination. For example, thimerosal. Adjuvants, to help stimulate a stronger immune response. For example, aluminum salts. Stabilizers, to keep the vaccine potent during transportation and storage. For example, sugars or gelatin. Others are residual trace amounts of materials that were used during the manufacturing process and removed. These can include: Cell culture materials, used to grow the vaccine antigens. For example, egg protein, various culture media. Inactivating ingredients, used to kill viruses or inactivate toxins. For example, formaldehyde. Antibiotics, used to prevent contamination by bacteria. For example, neomycin. The following table lists substances, other than active ingredients (i.e., antigens), shown in the manufacturers’ package insert (PI) as being contained in the final formulation of each vaccine. Note: Substances used in the manufacture of a vaccine but not listed as contained in the final product (e.g., culture media) can be found in each PI, but are not shown on this table. Each PI, which can be found on the FDA’s website (see below) contains a description of that vaccine’s manufacturing process, including the amount and purpose of each substance. In most PIs, this information is found -

General Properties of the Alkaline Phosphates: - Major Food and Technical Applications
Phosphorus Research Bulletin Vol. 15 (2004) p. 85-94 General Properties of the Alkaline Phosphates: - Major Food and Technical Applications P.HOURANT Deputy Business Line Manager, Prayon S.A., Business Unit Phosphates, Rue Joseph Wauters, 144 4480 Engis, Belgium; E-mail: [email protected] INTRODUCTION The alkaline phosphates are used for many food and technical applications. Phosphates have two characteristics that explain their four main properties: buffer agent, sequestering power, dispersing power and water holding capability. Those properties allow phosphates to be used in many food and technical applications. The main food applications are meat and seafood processing, baking and processed cheese, but others such as cereals, French fries, fruits and vegetables, beverages, noodles and so on also may need the use of phosphates. On the technical side, the main applications are the detergent products, the water treatment and the metal treatment. As for the food, many other applications require phosphates such as ceramics, bone china, paper and paints,... In meat products, phosphates salts interact in a unique way to bind water with proteins and improve the tenderness in meats. Treated products will maintain their juicy appearance as well as their natural nutritional properties texture and colour. In fish and seafood products, phosphates salts allow the retention of the natural juices of frozen fish fillets, prawns, shrimps, scallops and other seafood. Phosphates also help prevent the build-up of struvite crystals in tinned tuna and crabmeat. In processed cheese, phosphates are crucially important in the production of processed cheese. These products ensure a homogeneous and uniform melt of raw cheese and product stability. -
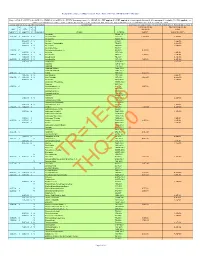
Composite Worker Air RSL May 2021 HQ10
Regional Screening Level (RSL) Composite Worker Ambient Air Table (TR=1E-06, HQ=1) May 2021 Key: I = IRIS; P = PPRTV; O = OPP; A = ATSDR; C = Cal EPA; X = PPRTV Screening Level; H = HEAST; W = TEF applied; E = RPF applied; G = user's guide Section 5; M = mutagen; V = volatile; R = RBA applied ; c = cancer; n = noncancer; * = where: n SL < 100X c SL; ** = where n SL < 10X c SL; SSL values are based on DAF=1; m = ceiling limit exceeded; s = Csat exceeded. Toxicity and Chemical-specific Information Contaminant Carcinogenic Target Risk (TR) = 1E-06 Noncancer Hazard Index (HI) = 1 k k v Carcinogenic SL Noncarcinogenic SL IUR e RfCi e o TR=1E-06 THI=1 (ug/m3)-1 y (mg/m3) y l mutagen Analyte CAS No. (ug/m3) (ug or fibers/m3) Acephate 30560-19-1 2.2E-06 I 9.0E-03 I V Acetaldehyde 75-07-0 5.6E+00 3.9E+01 Acetochlor 34256-82-1 3.1E+01 A V Acetone 67-64-1 1.4E+05 2.0E-03 X Acetone Cyanohydrin 75-86-5 8.8E+00 6.0E-02 I V Acetonitrile 75-05-8 2.6E+02 V Acetophenone 98-86-2 1.3E-03 C Acetylaminofluorene, 2- 53-96-3 9.4E-03 2.0E-05 I V Acrolein 107-02-8 8.8E-02 1.0E-04 I 6.0E-03 I M Acrylamide 79-06-1 1.2E-01 2.6E+01 1.0E-03 I V Acrylic Acid 79-10-7 4.4E+00 6.8E-05 I 2.0E-03 I V Acrylonitrile 107-13-1 1.8E-01 8.8E+00 6.0E-03 P Adiponitrile 111-69-3 2.6E+01 Alachlor 15972-60-8 Aldicarb 116-06-3 Aldicarb Sulfone 1646-88-4 Aldicarb sulfoxide 1646-87-3 4.9E-03 I V Aldrin 309-00-2 2.5E-03 1.0E-04 X V Allyl Alcohol 107-18-6 4.4E-01 6.0E-06 C 1.0E-03 I V Allyl Chloride 107-05-1 2.0E+00 4.4E+00 5.0E-03 P Aluminum 7429-90-5 2.2E+01 Aluminum Phosphide 20859-73-8 -
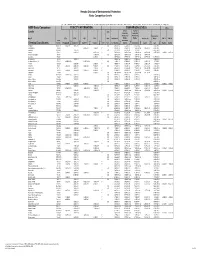
Basic Comparison Levels
Nevada Division of Environmental Protection Basic Comparison Levels Key: I=IRIS; P= PPRTV; N=NCEA; H=HEAST; A=ATSDR; O=Other Documents; CA=CalEPA S=Surrogate X=Appendix PPRTV E=Based on TEF scheme r=Route Extra Key: C = Cancer endpoint; N = Noncancer endpoint; sat = Saturation Limit; max = Ceiling Limit NDEP Basic Comparison TOXICITY INFORMATION COMPARISON LEVELS LBCLs Indoor Outdoor Levels Skin Industrial/ Industrial/ Commercial Commercial Residential May-17 SFo RfDo IUR RfCi Abs. Residential Worker Worker Ambient Air Water DAF 1 DAF 20 CAS w/o Dermal Chemical Constituents Number 1/(mg/kg-d) (mg/kg-d) (ug/m3)-1 (mg/m3) VOCc Soils Soil (mg/kg) (mg/kg) Soil (mg/kg) (µg/m3) (µg/l) (mg/kg) (mg/kg) Key Key key Key Key Key Key Key Key Acephate 30560-19-1 8.70E-03 I 4.00E-03 I 0.10 5.59E+01 C 7.52E+02 C 2.95E+02 C 7.73E+00 C Acetaldehyde 75-07-0 2.20E-06 I 9.00E-03 I V 1.23E+01 C 5.35E+01 C 1.00E+05 max 1.28E+00 C 2.55E+00 C Acetochlor 34256-82-1 2.00E-02 I 0.10 1.23E+03 N 4.67E+04 N 1.83E+04 N 6.67E+02 N Acetone 67-64-1 9.00E-01 I 3.10E+01 A V 7.04E+04 N 1.00E+05 max 1.00E+05 max 3.23E+04 N 2.05E+04 N 8.00E-01 1.60E+01 Acetone Cyanohydrin 75-86-5 2.00E-03 X 0.10 1.00E+05 max 1.00E+05 max 1.00E+05 max 2.09E+00 N Acetonitrile 75-05-8 6.00E-02 I V 1.00E+05 max 3.75E+03 N 1.00E+05 max 6.26E+01 N 1.25E+02 N Acetophenone 98-86-2 1.00E-01 I V 2.52E+03 sat 2.52E+03 sat 2.52E+03 sat 3.34E+03 N Acetylaminofluorene, 2- 53-96-3 3.80E+00 CA 1.30E-03 CA 0.10 1.28E-01 C 1.72E+00 C 6.75E-01 C 2.16E-03 C 1.77E-02 C Acrolein 107-02-8 5.00E-04 I 2.00E-05 I V -

Sodium Phosphate (Dibasic)
G-Biosciences, St Louis, MO, USA | 1-800-628-7730 | 1-314-991-6034 | [email protected] A Geno Technology, Inc. (USA) brand name Safety Data Sheet Cat. # RC-095 Sodium Phosphate (Dibasic) Size: 500g think proteins! think G-Biosciences! www.GBiosciences.com Sodium Phosphate (Dibasic) Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH) with its amendment Regulation (EU) 2015/830 Revision date: 5/11/2017 Version: 1.1 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product form : Substance Substance name : Sodium Phosphate (Dibasic) Chemical name : disodium hydrogenorthophosphate EC-No. : 231-448-7 CAS-No. : 7558-79-4 Product code : 250S Type of product : Pure substance,Hygroscopic substance. Preventive measures apply to the substance in dry state only Formula : Na2HPO4 Synonyms : ACETEST / anhydrous sodium acid phosphate / dibasic sodium phosphate / disodium acid phosphate / disodium acid phosphate, anhydrous / disodium hydrogen phosphate / disodium hydrogenorthophosphate / disodium hydrophosphate, anhydrous / disodium monohydrogen phosphate / disodium monohydrogen phosphate, anhydrous / disodium orthophosphate / disodium orthophosphate, anhydrous / disodium phosphate / disodium phosphate, anhydrous / disodium phosphoric acid / disodium phosphoric acid, anhydrous / DSP (=disodium phosphate) / DSP (=disodium phosphate), anhydrous / E339(b) food grade / exsiccated sodium phosphate / fema number 2398 / phosphate of soda / phosphoric acid disodium salt, anhydrous / phosphoric acid, disodium salt / secondary- sodium phosphate / secondary-sodium phosphate, anhydrous / secondary- sodiumorthophosphate, anhydrous / sec-sodium phosphate / soda phosphate / sodium hydrogen phosphate / sodium monohydrogen orthophosphate, anhydrous / sodium monohydrogen phosphate / sodium orthophosphate, mono-H / sodium orthophosphate, secondary- / sodium phosphate, dibasic / sodium phosphate, dibasic, anhydrous / sodium phosphate, exsiccated, anhydrous Product group : Raw material BIG No : 10787 1.2. -

Phosphates and Phosphoric Acid in Everyday Life
Detergents Metal treatment Fertilisers Water treatment Phosphates and phosphoric acid in everyday life Products to meet everyday needs PRAYON, a long tradition in the chemical industry Prayon was founded in 1982, but its roots reach back more than 100 years to the Liège region of Belgium. It is the parent company of the Prayon Group, a world leader in producing phosphate chemicals (fertilisers, detergents, food additives and technical applications) and developing inorganic chemical specialities. The Group’s expertise in the wet process production of phosphoric acid is acknowledged and utilised worldwide, including by its competitors. Currently, some 50% of phosphoric acid production sites use Prayon technology and 70% use Prayon equipment. Jointly owned by Office Chérifien des Phosphates (OCP) and Société Régionale d’Investissement de Wallonie (SRIW), the Group consists of over 20 companies located in more than 10 countries. It employs 1,450 people and generates a turnover of €500 million. Prayon boasts worldwide coverage via its production sites in Europe (Belgium and France), the Americas (USA, Brazil) and Africa (Morocco). The Group markets its products in more than 70 countries on five continents, an international presence that reinforces its position as a global player. PRAYON AND TECHNICAL PHOSPHATES Prayon markets the following major groups of phosphate products used in technical applications: • PURIFIED PHOSPHORIC ACID: produced using Prayon’s own solvent extraction process. Available in different grades and concentrations, this acid is used in a wide range of technical and food applications. Production sites: • Engis and Puurs (Belgium): 250,000 tonnes of P2O5 per year • Jorf-Lasfar (Morocco), via Emaphos, a joint venture between Prayon (33%), OCP (33%) and CFB (Chemische Fabrik Budenheim) (33%): 150,000 tonnes of P2O5 per year • Phosphate salts: sodium, potassium, calcium, ammonium and aluminium phosphates, and a wide variety of special blends based on these products. -

Disodium Hydrogen Phosphate
DISODIUM HYDROGEN PHOSPHATE Prepared at the 19th JECFA (1975), published in NMRS 55B (1976) and in FNP 52 (1992). Metals and arsenic specifications revised at the 59th JECFA (2002). A group MTDI of 70 mg/kg bw, as phosphorus from all food sources, was established at the 26th JECFA (1982) SYNONYMS Dibasic sodium phosphate, disodium phosphate, disodium acid phosphate, secondary sodium phosphate; INS No. 339(ii) DEFINITION Chemical names Disodium hydrogen phosphate, disodium hydrogen orthophosphate, disodium hydrogen monophosphate C.A.S. number 7558-79-4 Chemical formula Anhydrous: Na2HPO4 Hydrated: Na2HPO4 · x H2O Formula weight 141.98 (anhydrous) Assay Not less than 98.0% after drying DESCRIPTION Anhydrous: White, hygroscopic, odourless powder Dihydrate: White crystalline, odourless solid Heptahydrate: White, odourless, efflorescent crystals or granular powder Dodecahydrate: White, efflorescent, odourless powder or crystals FUNCTIONAL USES Emulsifier, texturizer, buffer CHARACTERISTICS IDENTIFICATION Solubility (Vol. 4) Freely soluble in water; insoluble in ethanol pH (Vol. 4) 9.0- 9.6 (1 in 100 soln) Test for sodium (Vol. 4) Passes test Test for phosphate (Vol. 4) Passes test Test for orthophosphate Dissolve 0.1 g of the sample in 10 ml water, acidify slightly with dilute (Vol. 4) acetic acid TS, and add 1 ml of silver nitrate TS. A yellow precipitate is formed. PURITY Loss on drying (Vol. 4) Anhydrous: Not more than 5.0% (40o, 3 h, then 105o, 5 h) Dihydrate: Not more than 22.0% (40o, 3 h, then 105o, 5 h) Heptahydrate: Not more than 50.0% (40o, 3 h, then 105o, 5 h) Dodecahydrate: Not more than 61.0% (40o, 3 h, then 105o, 5 h) Water insoluble substances Not more than 0.2% (Vol. -

The Estimation of Acidic Behavior of Wood by Treatment with Aqueous Na2hpo4 Solution
Hindawi Publishing Corporation Journal of Analytical Methods in Chemistry Volume 2012, Article ID 496305, 9 pages doi:10.1155/2012/496305 Research Article The Estimation of Acidic Behavior of Wood by Treatment with Aqueous Na2HPO4 Solution Gunes¨ ¸Uc¸ar and Mualla Balaban Uc¸ar Faculty of Forestry of Istanbul University, Bahc¸ekoy,¨ 80895 Istanbul, Turkey Correspondence should be addressed to Gunes¨ ¸Uc¸ar, [email protected] Received 4 November 2011; Revised 24 December 2011; Accepted 30 December 2011 Academic Editor: Mohamed Mohamed Copyright © 2012 G. Uc¸ar and M. Balaban Uc¸ar. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. As a new approach, the acidity that wood exhibits under moderate conditions is assayed by stimulated dissociation of weak wood acids in lightly basic secondary phosphate solutions. To assure a sufficient dissociation of hardly soluble weak acids in the solution, the amount of wood suspended in Na2HPO4 solutions should be small but vary depending on the degree of acidity of wood species. However, the difficulties are associated with the titration of very dilute acids limiting the precision of the measurement. If the disintegrated wood is suspended in a secondary phosphate solution, the weak woods acids form the conjugate acid Na2HPO4 from secondary phosphate Na2HPO4 resulting in a pH fall of the solution. The decrease in the pH value in phosphate solution, which depends on the wood acidity, can be evaluated to estimate the acidity arising from wood under moderate conditions. -

Food and Non-Food Uses of Phosphates Tony Gichuhi, Phd; Global R&D Director, ICL Phosphate Specialty Paola Campo; Applications Development Manager, Prayon Inc
The Phosphate Forum of the Americas & The International Food Additives Council Presents Phosphates in Our World: Food and Non-Food Uses of Phosphates Tony Gichuhi, PhD; Global R&D Director, ICL Phosphate Specialty Paola Campo; Applications Development Manager, Prayon Inc. Taylor Wallace, PhD, CFS, FACN; Think Healthy Group, Inc. and George Mason University Tuesday, March 3, 2020 Phosphate Forum of the Americas Introduction Moderator: Berit Dockter MPP, RD, LD Scientific and Regulatory Affairs Manager International Food Additives Council Phosphate Forum of the Americas Technical Difficulties: Call Holly at 1-847-686-2347 Q&A: Input questions into the “ask a question box” Contact: Phosphate Forum of the Americas [email protected] About the Phosphate Forum of the Americas • The Phosphate Forum of the Americas (PFA) was established in 1996 to facilitate the exchange of information on matters relating to inorganic phosphates. • The mission of the Phosphate Forum of the Americas is to provide educational information on scientific and technical matters relating to inorganic phosphate products, and assess the role of activities progressing toward a sustainable environment. • Visit phosphatesfacts.org Phosphate Forum of the Americas About the International Food Additives Council • The International Food Additives Council (IFAC) is a global association representing manufacturers and end-users of food ingredients, including food additives. • IFAC strives to promote science-based regulations, standards and specifications for food ingredients worldwide. • The IFAC mission is to be the recognized global leader for food ingredient information, education and science, advocating for sound food ingredient policies. • Visit: foodingredientfacts.org Phosphate Forum of the Americas Objectives of the Webinar • Be able to identify at least one indication for the use of phosphates in food and use in non-food products. -

Reducing the Level of Added Disodium Phosphate Alters the Chemical and Physical Properties of Processed Cheese Timothy Guinee, Brendan O’Kennedy
Reducing the level of added disodium phosphate alters the chemical and physical properties of processed cheese Timothy Guinee, Brendan O’Kennedy To cite this version: Timothy Guinee, Brendan O’Kennedy. Reducing the level of added disodium phosphate alters the chemical and physical properties of processed cheese. Dairy Science & Technology, EDP sci- ences/Springer, 2012, 92 (5), pp.469-486. 10.1007/s13594-011-0053-2. hal-00930613 HAL Id: hal-00930613 https://hal.archives-ouvertes.fr/hal-00930613 Submitted on 1 Jan 2012 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Dairy Sci. & Technol. (2012) 92:469–486 DOI 10.1007/s13594-011-0053-2 ORIGINAL PAPER Reducing the level of added disodium phosphate alters the chemical and physical properties of processed cheese Timothy P. Guinee & Brendan T. O’Kennedy Received: 21 July 2011 /Revised: 6 December 2011 /Accepted: 8 December 2011 / Published online: 15 February 2012 # INRA and Springer-Verlag, France 2011 Abstract The intake of sodium in modern western diet is excessive, with potentially harmful effects on health. Consequently, there is a drive to reduce the sodium content of foods. A major source of sodium in processed cheeses (PCs) is the added emulsifying salts (sodium phosphates or citrates) which play a key role in product formation and stabilisation.