Fluoride-Containing Toothpastes Available in Two European Countries
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemicals Used for Chemical Manufacturing Page 1 of 2
Chemicals used for Chemical Manufacturing Page 1 of 2 Acetic Acid (Glacial, 56%) Glycol Ether PMA Acetone Glycol Ether PNB Acrylic Acid Glycol Ether PNP Activated Carbon Glycol Ether TPM Adipic Acid Glycols Aloe Vera Grease Aluminum Stearate Gum Arabic Aluminum Sulfate Heat Transfer Fluids Amino Acid Heptane Ammonium Acetate Hexane Ammonium Bicarbonate Hydrazine Hydrate Ammonium Bifluoride Hydrochloric Acid (Muriatic) Ammonium Chloride Hydrogen Peroxide Ammonium Citrate Hydroquinone Ammonium Hydroxide Hydroxylamine Sulfate Ammonium Laureth Sulfate Ice Melter Ammonium Lauryl Sulfate Imidazole Ammonium Nitrate Isobutyl Acetate Ammonium Persulfate Isobutyl Alcohol Ammonium Silicofluoride Calcium Stearate Dipropylene Glycol Isopropanolamine Ammonium Sulfate Carboxymethylcellulose Disodium Phosphate Isopropyl Acetate Antifoams Caustic Potash D'Limonene Isopropyl Alcohol Antifreeze Caustic Soda (All Grades) Dodecylbenzene Sulfonic Acid Isopropyl Myristate Antimicrobials Caustic Soda (Beads, Prills) (DDBSA) Isopropyl Palmitate Antimony Oxide Cetyl Alcohol Dowfrost Itaconic Acid Aqua Ammonia Cetyl Palmitate Dowfrost HD Jojoba Oil Ascorbic Acid Chlorine, Granular Dowtherm SR-1 Keratin Barium Carbonate Chloroform Dowtherm 4000 Lactic Acid Barium Chloride Chromic Acid EDTA Lanolin Beeswax Citric Acid (Dry and Liquid) EDTA Plus Lauric Acid Bentonite Coal Epsom Salt Lauryl Alcohol Benzaldehyde Cocamide DEA Ethyl Acetate Lecithin Benzoic Acid Copper Nitrate Ethyl Alcohol (Denatured) Lime Benzyl Alcohol Copper Sulfate Ethylene Glycol Linoleic Acid Bicarbonate -

Bactericide-Disinfectant
, r f.~ n,r.~il·'\L H.. ~t.t;I" "" . i ltM)"ft . "' .' .,.,,~: .. ;;:.~ ,.;·~~~t:.:.· ,.J.~ . ... --' "'.~.};" ,",>;"'!:,., ~ " .. ". , ; IlUNGtCIOE' MID RCOfNTlCtOI .. ~.' ".' • ., . <",~. .. ,'. 1 ~Of: ~~Itr. ,<"{}~ _ t EO IJ"OS~A C :J-) l - (BACTERICIDE -DISINFECTANT) DESC Rlt.JllC"N: P''1k'or is a nor-sudsing, chlorinated, perman a solution can be recommended for dairy utensils, milking ma 4. For home l gana·ed o~tprger.t F'owaE'r for q'Jick, efficient cleaning, disin chines and equipment. The same solution can be us~d on dairy water for dis fecting, sani rizing and (JE:odorizir,g. It is designed for use on all farm utensils as well as washing cows' udders and milkr.:rs' utensils. For c11 types of equipment and utensils that are used in the handling hands. surfaces such of food products, milk, meats, beverages, etc. Authorized for ounce per que use under the U. S. Department of Agriculture Consumer and 2. Pinklor can be used very effec1ively in food and beveraqe Marketing Service Inspection Programs for poultry, meat, milk plants as a disinfectant for equipment using a solution of 1 and egg product plants. F oer 40 gallons of water and applying this solution in DANGI c.. i.:,.'r that insures conto(t '/./ith all po "ts and surfaces. OREN. AVOI Specifications 0 STUFFS. HAF Active: Trisodium Phosphate (hydrated) over 91.00 0 3. Where local and state regulations are in effect, consu:t Sodium Hypochlorite over 3.500 0 rules of you" local authorities tor volume of chlorine required SKIN OR IN 0 Sodium Lauryl Sulfate under 0.05 0 in rinse solutil'ns. -

Safety Data Sheet TSP - TRISODIUM PHOSPHATE
Safety Data Sheet TSP - TRISODIUM PHOSPHATE Date of Revision: 2/11/2015 Section 1 – Chemical Product and Company Identification Product/Chemical Name: Trisodium Phosphate dodecahydrate Chemical Formula: Na3PO4*12H2O CAS Number: 10101-89-0 Other Designations: TSP; trisodium orthophosphate; tribasic; tertiary sodium phosphate; trisodium phosphate Derivation: Prepared by combining proper proportions of phosphoric acid and soda to form disodium phosphate, then adding a caustic soda Supplied by: PRO Chemical & Dye 126 Shove Street Fall River, MA 02724 Emergency Telephone Numbers: 800-255-3924 ChemTel. (United States) + 1 01 813-248-0585 (Outside the United States) 1. Section 2 - Hazards Identification HMIS ***** Emergency Overview ***** H 3 MAY CAUSE EYE INJURY. CAUSES SKIN IRRITATlON. MAYBE HARMFUL IF SWALLOWED. F 0 Potential Health Effects R 0 PPE Primary Entry Routes: Inhalation, ingestion or skin contact. Sec. 8 Target Organs: Skin, digestive tract. HAZARDS IDENTIFICATION Classification of the substance or mixture GHS Classification in accordance with 29 CFR 1910 (OSHA DCS) Skin corrosion (Category I B). H314 Serious eye damage (Category I). H318 GHS Label elements, including precautionary statements Pictogram Signal word Danger Hazard statement(s) H314 Causes severe skin burns and eye damage. Precautionary Statement(s) P260 Do not breathe dust or mist. P264 Wash skin thoroughly after handling. P280 Wear protective gloves protective clothing/ eye protection/ face protection. P301 + P330 + P331 IF SWALLOWED: rinse mouth. DO NOT induce vomiting. P303 + P36l + P353 IF ON SKIN (or hair): Remove. Take off immediately all contaminated clothing. Rinse skin with water / shower. P304 + P340 IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing. -

CX/FA 18/50/5 January 2018 JOINT FAO/WHO FOOD STANDARDS
E Agenda Item 4 (a) CX/FA 18/50/5 January 2018 JOINT FAO/WHO FOOD STANDARDS PROGRAMME CODEX COMMITTEE ON FOOD ADDITIVES Fiftieth Session ENDORSEMENT AND/OR REVISION OF MAXIMUM LEVELS FOR FOOD ADDITIVES AND PROCESSING AIDS IN CODEX STANDARDS BACKGROUND 1. In accordance with the section concerning Relations between Commodity Committees and General Committees of the Codex Alimentarius Commission Procedural Manual, “All provisions in respect of food additives (including processing aids) contained in Codex commodity standards should be referred to the Committee on Food Additives, preferably before the Standards have been advanced to Step 5 of the Procedure for the Elaboration of Codex Standards or before they are considered by the commodity committee concerned at Step 7, though such referral should not be allowed to delay the progress of the Standard to the subsequent Steps of the Procedure.”. 2. The following food additive and processing aids provisions of Codex standards have been submitted for endorsement since the 49th Session of the Codex Committee on Food Additives and are listed by: (i) Technological function, INS number and food additive name; (ii) Maximum level; (iii) ADI (mg additive/kg body weight per day); and (iv) Notes. 3. The following abbreviations have been used in the preparation of this paper: INS International Numbering System for food additives. The INS (INS) is intended as a harmonised naming system for food additives as an alternative to the use of the specific name, which may be lengthy1. ADI Acceptable Daily Intake. An estimate of the amount of a substance in food or drinking-water, expressed on a body-weight basis, that can be ingested daily over a lifetime without appreciable risk (standard human = 60 kg)2. -

Brochure-Product-Range.Pdf
PRODUCT RANGE 2015 edition ANSI Standard 60 NSF® CERTIFIED HALAL M ISLAMIC FOOD AND NUTRITION ® COUNCIL OF AMERICA Rue Joseph Wauters, 144 ISO 9001:2008 (Quality) / OHSAS 18001:2007 (Health/ B-4480 Engis Safety) / ISO 14001:2004 (Environment) / ISO 22000:2005 www.globulebleu.com (Food Safety) / FSSC 22000:2013 (Food Safety). Tel. +32 (0) 4 273 93 58 Our food grade phosphates are allergen free, GMO free, Fax. +32 (0) 4 275 68 36 BSE/TSE free. www.prayon.com mail. [email protected] Design by www.prayon.com PRODUCT RANGE | 11 TABLE OF CONTENTS HORTICULTURE APPLICATIONS HORTIPRAY® RANGE FOR HORTICULTURE* FOOD AND INDUSTRIAL APPLICATIONS PRODUCT NAME Bulk density P O pH N-NH Made 2 5 4 MONOAMMONIUM PHOSPHATE - NH4H2PO4 in 3 3 % 1% % Sodium orthophosphates ................................................................................... 03 g/cm lbs/ft indicative indicative indicative Water-soluble fertilisers. Sodium pyrophosphates .................................................................................... 04 HORTIPRAY® MAP Horticultural Grade 0.9 56 61 4.5 12 Sodium tripolyphosphates ................................................................................. 05 HORTIPRAY® MAP 12.60 Horticultural Grade 0.9 56 60 5 12.1 Water-soluble fertilisers; Sodium polyphosphates ..................................................................................... 06 HORTIPRAY® MAP anticalc Horticultural Grade 0.9 56 61 4.5 12 preventive action against clogging. Potassium orthophosphates ............................................................................. -

Simple Chemical Experiments Simple Chemical Experiments
SIMPLE CHEMICAL EXPERIMENTS SIMPLE CHEMICAL EXPERIMENTS By ALFRED MORGAN Illustrated by THE AUTHOR APPLETON-CENTURY-CROFTS, INC. NEW YORK COPYRIGHT, 1941, BY D. APPLETON-CENTURY COMPANY, INC All rights reserved. This book, or parts thereof, must not be reproduced in any form without permission of the publisher. PRINTED IN THE UNITED STATES OF AMERICA CONTENTS CHAPTER PAGE I. YOUR LABORATORY i II. EXPERIMENTS WITH PRECIPITATES .... 26 III. EXPERIMENTS WITH SULFUR AND SOME OF ITS COMPOUNDS 54 IV. EXPERIMENTS WITH OXYGEN AND OXYGEN COM POUNDS 73 V. EXPERIMENTS WITH GASES AND SOME OF THEIR COMPOUNDS 103 VI. CHEMICAL TESTS 123 VII. SAFE "FIREWORKS" 144 VIII. EXPERIMENTS WITH A FEW ORGANIC COMPOUNDS 156 IX. CHEMICAL TRICKS AND MAGIC 170 X. MISCELLANEOUS EXPERIMENTS 186 XI. PRACTICAL USES FOR YOUR CHEMICAL KNOWL EDGE 214 XII. THE CHEMICALS YOU WILL NEED . .231 INDEX OF CHEMICALS 259 GENERAL INDEX 263 V SIMPLE CHEMICAL EXPERIMENTS *> CHAPTER I I YOUR LABORATORY I OST of the experiments described in this book can be M performed without elaborate equipment or apparatus. | For them you will need only a few bottles, test-tubes, meas- i uring-spoons, and an alcohol lamp. Jelly glasses, mayonnaise f jars, small enameled saucepans, and thin glass tumblers can | often be substituted for the beakers, flasks, and glassware of I the professional chemist. t A few of the experiments require beakers, flasks, tubing, | funnels, filter paper, crucibles, mortar and pestle, and Bunsen I burner. The small sizes of these are not expensive. Frequently the cost of apparatus and chemicals can be shared by estab lishing a "community" laboratory which is used by two or more experimenters. -

Vaccine Excipient Table
Vaccine Excipient Summary Excipients Included in U.S. Vaccines, by Vaccine In addition to weakened or killed disease antigens (viruses or bacteria), vaccines contain very small amounts of other ingredients – excipients. Some excipients are added to a vaccine for a specific purpose. These include: Preservatives, to prevent contamination. For example, thimerosal. Adjuvants, to help stimulate a stronger immune response. For example, aluminum salts. Stabilizers, to keep the vaccine potent during transportation and storage. For example, sugars or gelatin. Others are residual trace amounts of materials that were used during the manufacturing process and removed. These can include: Cell culture materials, used to grow the vaccine antigens. For example, egg protein, various culture media. Inactivating ingredients, used to kill viruses or inactivate toxins. For example, formaldehyde. Antibiotics, used to prevent contamination by bacteria. For example, neomycin. The following table lists substances, other than active ingredients (i.e., antigens), shown in the manufacturers’ package insert (PI) as being contained in the final formulation of each vaccine. Note: Substances used in the manufacture of a vaccine but not listed as contained in the final product (e.g., culture media) can be found in each PI, but are not shown on this table. Each PI, which can be found on the FDA’s website (see below) contains a description of that vaccine’s manufacturing process, including the amount and purpose of each substance. In most PIs, this information is found -

(12) United States Patent (10) Patent No.: US 8,815,911 B2 Pettersson Et Al
US00881.5911B2 (12) United States Patent (10) Patent No.: US 8,815,911 B2 Pettersson et al. (45) Date of Patent: Aug. 26, 2014 (54) ALFENTANIL COMPOSITION FOR THE WO WO-OOf 16751 A1 3, 2000 TREATMENT OF ACUTE PAIN WO WO-OOf 51539 A1 9, 2000 WO WO-01/30288 A1 5, 2001 WO WO-O2/O67903 A2 9, 2002 (71) Applicant: Orexo AB, Uppsala (SE) WO WOO3,OO5944 A1 1, 2003 WO WO-2004/067004 A1 8, 2004 (72) Inventors: Anders Pettersson, Kode (SE); Barbro WO WO-2006/097361 A1 9, 2006 Johansson, Uppsala (SE); Emil WO WO-2006,103418 A1 10, 2006 Schwan, Uppsala (SE) WO WO-2007/081948 A2 7, 2007 s WO WO-2007/081949 A2 7/2007 WO WO-2007/141328 A1 12/2007 (73) Assignee: Orexo AB, Uppsala (SE) WO WO-2008/068471 A1 6, 2008 - WO WO-2008/085765 A2 7, 2008 (*) Notice: Subject to any disclaimer, the term of this WO WO-2008, 106689 A2 9, 2008 patent is extended or adjusted under 35 W. W838887} h A. 5,588 U.S.C. 154(b) by 0 days. WO WO-2010/059504 A1 5, 2010 WO WO-2010, 132605 A1 11, 2010 (21) Appl. No.: 13/874,762 WO WO-2010/141505 A1 12/2010 WO WO-2011/O17484 A2 2, 2011 (22) Filed: May 1, 2013 WO WO-2011/057199 A1 5, 2011 (65) Prior Publication Data OTHER PUBLICATIONS US 2014/OOO5223 A1 Jan. 2, 2014 FDA Guidance for Industry. Orally Disintegrating Tablets; Dec. 2008. (30) Foreign Application Priority Data Mohanachandran et al. -

General Properties of the Alkaline Phosphates: - Major Food and Technical Applications
Phosphorus Research Bulletin Vol. 15 (2004) p. 85-94 General Properties of the Alkaline Phosphates: - Major Food and Technical Applications P.HOURANT Deputy Business Line Manager, Prayon S.A., Business Unit Phosphates, Rue Joseph Wauters, 144 4480 Engis, Belgium; E-mail: [email protected] INTRODUCTION The alkaline phosphates are used for many food and technical applications. Phosphates have two characteristics that explain their four main properties: buffer agent, sequestering power, dispersing power and water holding capability. Those properties allow phosphates to be used in many food and technical applications. The main food applications are meat and seafood processing, baking and processed cheese, but others such as cereals, French fries, fruits and vegetables, beverages, noodles and so on also may need the use of phosphates. On the technical side, the main applications are the detergent products, the water treatment and the metal treatment. As for the food, many other applications require phosphates such as ceramics, bone china, paper and paints,... In meat products, phosphates salts interact in a unique way to bind water with proteins and improve the tenderness in meats. Treated products will maintain their juicy appearance as well as their natural nutritional properties texture and colour. In fish and seafood products, phosphates salts allow the retention of the natural juices of frozen fish fillets, prawns, shrimps, scallops and other seafood. Phosphates also help prevent the build-up of struvite crystals in tinned tuna and crabmeat. In processed cheese, phosphates are crucially important in the production of processed cheese. These products ensure a homogeneous and uniform melt of raw cheese and product stability. -

Navy Boiler Water Chemical Tests and Treatments
Experiment 14D Final 2/2015 NAVY BOILER WATER CHEMICAL TESTS AND TREATMENTS MATERIALS: Automatic zero burets, 100 and 10 mL graduated cylinders, 400 mL beaker, magnetic stir bar, 50-mL buret, evaporating dish, two 100 mL volumetric flasks, 250 mL Erlenmeyer flasks, boiler water, 0.010 M HNO3, 0.050 M HNO3, 0.0050 M Hg(NO3)2, 0.0200 M Na2S2O3, 2.0 M MnSO4, KOH – KI solution, 0.060 M Na2SO3 (must be fresh), 18 M H2SO4, 2% starch solution, phenolphthalein, methyl purple, chloride indicator, pH meter, thermometer, magnetic stirrer; conductivity meter. PURPOSE: The purpose of this experiment is to familiarize the student with the chemical tests used for Navy boiler water systems, and to demonstrate the chemical principles associated with some of the tests and treatments used for these critical systems. These tests are used aboard ships to monitor the quality of the feed water to prevent scale formation and corrosion. LEARNING OBJECTIVES: By the end of this experiment, the student should be able to demonstrate the following proficiencies: 1. Explain the use of buffers in boiler water treatment. 2. Explain the process of an acid/base titration and the use of indicators. 3. Standardize and use a pH meter. 4. Convert concentration units of molarity to ppm (parts per million). 5. Explain the role of chloride ion testing and conductivity monitoring for boiler water. 6. Describe a chemical treatment for removing dissolved oxygen from water. PRE-LAB: Complete the pre-lab prior to lab. DISCUSSION - NAVY BOILER WATER TESTS All Naval vessels use boilers to prepare steam. -
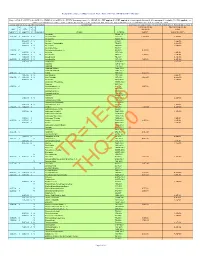
Composite Worker Air RSL May 2021 HQ10
Regional Screening Level (RSL) Composite Worker Ambient Air Table (TR=1E-06, HQ=1) May 2021 Key: I = IRIS; P = PPRTV; O = OPP; A = ATSDR; C = Cal EPA; X = PPRTV Screening Level; H = HEAST; W = TEF applied; E = RPF applied; G = user's guide Section 5; M = mutagen; V = volatile; R = RBA applied ; c = cancer; n = noncancer; * = where: n SL < 100X c SL; ** = where n SL < 10X c SL; SSL values are based on DAF=1; m = ceiling limit exceeded; s = Csat exceeded. Toxicity and Chemical-specific Information Contaminant Carcinogenic Target Risk (TR) = 1E-06 Noncancer Hazard Index (HI) = 1 k k v Carcinogenic SL Noncarcinogenic SL IUR e RfCi e o TR=1E-06 THI=1 (ug/m3)-1 y (mg/m3) y l mutagen Analyte CAS No. (ug/m3) (ug or fibers/m3) Acephate 30560-19-1 2.2E-06 I 9.0E-03 I V Acetaldehyde 75-07-0 5.6E+00 3.9E+01 Acetochlor 34256-82-1 3.1E+01 A V Acetone 67-64-1 1.4E+05 2.0E-03 X Acetone Cyanohydrin 75-86-5 8.8E+00 6.0E-02 I V Acetonitrile 75-05-8 2.6E+02 V Acetophenone 98-86-2 1.3E-03 C Acetylaminofluorene, 2- 53-96-3 9.4E-03 2.0E-05 I V Acrolein 107-02-8 8.8E-02 1.0E-04 I 6.0E-03 I M Acrylamide 79-06-1 1.2E-01 2.6E+01 1.0E-03 I V Acrylic Acid 79-10-7 4.4E+00 6.8E-05 I 2.0E-03 I V Acrylonitrile 107-13-1 1.8E-01 8.8E+00 6.0E-03 P Adiponitrile 111-69-3 2.6E+01 Alachlor 15972-60-8 Aldicarb 116-06-3 Aldicarb Sulfone 1646-88-4 Aldicarb sulfoxide 1646-87-3 4.9E-03 I V Aldrin 309-00-2 2.5E-03 1.0E-04 X V Allyl Alcohol 107-18-6 4.4E-01 6.0E-06 C 1.0E-03 I V Allyl Chloride 107-05-1 2.0E+00 4.4E+00 5.0E-03 P Aluminum 7429-90-5 2.2E+01 Aluminum Phosphide 20859-73-8 -
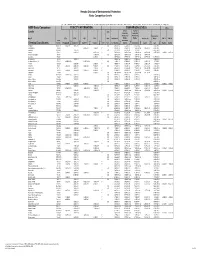
Basic Comparison Levels
Nevada Division of Environmental Protection Basic Comparison Levels Key: I=IRIS; P= PPRTV; N=NCEA; H=HEAST; A=ATSDR; O=Other Documents; CA=CalEPA S=Surrogate X=Appendix PPRTV E=Based on TEF scheme r=Route Extra Key: C = Cancer endpoint; N = Noncancer endpoint; sat = Saturation Limit; max = Ceiling Limit NDEP Basic Comparison TOXICITY INFORMATION COMPARISON LEVELS LBCLs Indoor Outdoor Levels Skin Industrial/ Industrial/ Commercial Commercial Residential May-17 SFo RfDo IUR RfCi Abs. Residential Worker Worker Ambient Air Water DAF 1 DAF 20 CAS w/o Dermal Chemical Constituents Number 1/(mg/kg-d) (mg/kg-d) (ug/m3)-1 (mg/m3) VOCc Soils Soil (mg/kg) (mg/kg) Soil (mg/kg) (µg/m3) (µg/l) (mg/kg) (mg/kg) Key Key key Key Key Key Key Key Key Acephate 30560-19-1 8.70E-03 I 4.00E-03 I 0.10 5.59E+01 C 7.52E+02 C 2.95E+02 C 7.73E+00 C Acetaldehyde 75-07-0 2.20E-06 I 9.00E-03 I V 1.23E+01 C 5.35E+01 C 1.00E+05 max 1.28E+00 C 2.55E+00 C Acetochlor 34256-82-1 2.00E-02 I 0.10 1.23E+03 N 4.67E+04 N 1.83E+04 N 6.67E+02 N Acetone 67-64-1 9.00E-01 I 3.10E+01 A V 7.04E+04 N 1.00E+05 max 1.00E+05 max 3.23E+04 N 2.05E+04 N 8.00E-01 1.60E+01 Acetone Cyanohydrin 75-86-5 2.00E-03 X 0.10 1.00E+05 max 1.00E+05 max 1.00E+05 max 2.09E+00 N Acetonitrile 75-05-8 6.00E-02 I V 1.00E+05 max 3.75E+03 N 1.00E+05 max 6.26E+01 N 1.25E+02 N Acetophenone 98-86-2 1.00E-01 I V 2.52E+03 sat 2.52E+03 sat 2.52E+03 sat 3.34E+03 N Acetylaminofluorene, 2- 53-96-3 3.80E+00 CA 1.30E-03 CA 0.10 1.28E-01 C 1.72E+00 C 6.75E-01 C 2.16E-03 C 1.77E-02 C Acrolein 107-02-8 5.00E-04 I 2.00E-05 I V