Commission Directive 2002/82/EC Amending Directive 96
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Safety Data Sheet TSP - TRISODIUM PHOSPHATE
Safety Data Sheet TSP - TRISODIUM PHOSPHATE Date of Revision: 2/11/2015 Section 1 – Chemical Product and Company Identification Product/Chemical Name: Trisodium Phosphate dodecahydrate Chemical Formula: Na3PO4*12H2O CAS Number: 10101-89-0 Other Designations: TSP; trisodium orthophosphate; tribasic; tertiary sodium phosphate; trisodium phosphate Derivation: Prepared by combining proper proportions of phosphoric acid and soda to form disodium phosphate, then adding a caustic soda Supplied by: PRO Chemical & Dye 126 Shove Street Fall River, MA 02724 Emergency Telephone Numbers: 800-255-3924 ChemTel. (United States) + 1 01 813-248-0585 (Outside the United States) 1. Section 2 - Hazards Identification HMIS ***** Emergency Overview ***** H 3 MAY CAUSE EYE INJURY. CAUSES SKIN IRRITATlON. MAYBE HARMFUL IF SWALLOWED. F 0 Potential Health Effects R 0 PPE Primary Entry Routes: Inhalation, ingestion or skin contact. Sec. 8 Target Organs: Skin, digestive tract. HAZARDS IDENTIFICATION Classification of the substance or mixture GHS Classification in accordance with 29 CFR 1910 (OSHA DCS) Skin corrosion (Category I B). H314 Serious eye damage (Category I). H318 GHS Label elements, including precautionary statements Pictogram Signal word Danger Hazard statement(s) H314 Causes severe skin burns and eye damage. Precautionary Statement(s) P260 Do not breathe dust or mist. P264 Wash skin thoroughly after handling. P280 Wear protective gloves protective clothing/ eye protection/ face protection. P301 + P330 + P331 IF SWALLOWED: rinse mouth. DO NOT induce vomiting. P303 + P36l + P353 IF ON SKIN (or hair): Remove. Take off immediately all contaminated clothing. Rinse skin with water / shower. P304 + P340 IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing. -

Brochure-Product-Range.Pdf
PRODUCT RANGE 2015 edition ANSI Standard 60 NSF® CERTIFIED HALAL M ISLAMIC FOOD AND NUTRITION ® COUNCIL OF AMERICA Rue Joseph Wauters, 144 ISO 9001:2008 (Quality) / OHSAS 18001:2007 (Health/ B-4480 Engis Safety) / ISO 14001:2004 (Environment) / ISO 22000:2005 www.globulebleu.com (Food Safety) / FSSC 22000:2013 (Food Safety). Tel. +32 (0) 4 273 93 58 Our food grade phosphates are allergen free, GMO free, Fax. +32 (0) 4 275 68 36 BSE/TSE free. www.prayon.com mail. [email protected] Design by www.prayon.com PRODUCT RANGE | 11 TABLE OF CONTENTS HORTICULTURE APPLICATIONS HORTIPRAY® RANGE FOR HORTICULTURE* FOOD AND INDUSTRIAL APPLICATIONS PRODUCT NAME Bulk density P O pH N-NH Made 2 5 4 MONOAMMONIUM PHOSPHATE - NH4H2PO4 in 3 3 % 1% % Sodium orthophosphates ................................................................................... 03 g/cm lbs/ft indicative indicative indicative Water-soluble fertilisers. Sodium pyrophosphates .................................................................................... 04 HORTIPRAY® MAP Horticultural Grade 0.9 56 61 4.5 12 Sodium tripolyphosphates ................................................................................. 05 HORTIPRAY® MAP 12.60 Horticultural Grade 0.9 56 60 5 12.1 Water-soluble fertilisers; Sodium polyphosphates ..................................................................................... 06 HORTIPRAY® MAP anticalc Horticultural Grade 0.9 56 61 4.5 12 preventive action against clogging. Potassium orthophosphates ............................................................................. -

Ionic Liquid + Biomolecule
Sónia Isabel Pereira Branco Licenciatura em Ciências da Engenharia Química e Bioquímica Aqueous Biphasic System based on Cholinium Ionic Liquids: Extraction of Biologically Active Phenolic Acids Dissertação para obtenção do Grau de Mestre em Engenharia Química e Bioquímica Orientador: Doutora Isabel Maria Delgado Jana Marrucho Ferreira, Investigadora Coordenadora, Laboratório de Termodinâmica Molecular, ITQB-UNL Presidente: Doutora Susana Filipe Barreiros Arguente: Doutor Alexandre Babo de Almeida Paiva Vogal: Doutora Isabel Maria Delgado Jana Marrucho Ferreira Setembro 2014 II UNIVERSIDADE NOVA DE LISBOA Faculdade de Ciências e Tecnologia Departamento de Química Aqueous Biphasic System based on Cholinium Ionic Liquids: Extraction of Biologically Active Phenolic Acids Sónia Isabel Pereira Branco Dissertação apresentada na Faculdade de Ciências e Tecnologia da Universidade Nova de Lisboa para obtenção do grau Mestre em Engenharia Química e Bioquímica Orientadores: Doutora Isabel Maria Delgado Jana Marrucho Ferreira 2014 III IV Aqueous Biphasic Systems based on Cholinium Ionic Liquids: Extraction of Biologically Active Phenolic Acids COPYRIGHT Sónia Isabel Pereira Branco Faculdade de Ciências e Tecnologia Universidade Nova de Lisboa A Faculdade de Ciências e Tecnologia e a Universidade Nova de Lisboa têm o direito, perpétuo e sem limites geográficos, de arquivar e publicar esta dissertação através de exemplares impressos reproduzidos em papel ou de forma digital, ou por qualquer outro meio conhecido ou que venha a ser inventado, e de a divulgar através de repositórios científicos e de admitir a sua cópia e distribuição com objectivos educacionais ou de investigação, não comerciais, desde que seja dado crédito ao autor e editor. V VI Agradecimentos Durante a realização desta tese, contei com o apoio de várias pessoas sem as quais não teria concluído esta etapa. -

Vaccine Excipient Table
Vaccine Excipient Summary Excipients Included in U.S. Vaccines, by Vaccine In addition to weakened or killed disease antigens (viruses or bacteria), vaccines contain very small amounts of other ingredients – excipients. Some excipients are added to a vaccine for a specific purpose. These include: Preservatives, to prevent contamination. For example, thimerosal. Adjuvants, to help stimulate a stronger immune response. For example, aluminum salts. Stabilizers, to keep the vaccine potent during transportation and storage. For example, sugars or gelatin. Others are residual trace amounts of materials that were used during the manufacturing process and removed. These can include: Cell culture materials, used to grow the vaccine antigens. For example, egg protein, various culture media. Inactivating ingredients, used to kill viruses or inactivate toxins. For example, formaldehyde. Antibiotics, used to prevent contamination by bacteria. For example, neomycin. The following table lists substances, other than active ingredients (i.e., antigens), shown in the manufacturers’ package insert (PI) as being contained in the final formulation of each vaccine. Note: Substances used in the manufacture of a vaccine but not listed as contained in the final product (e.g., culture media) can be found in each PI, but are not shown on this table. Each PI, which can be found on the FDA’s website (see below) contains a description of that vaccine’s manufacturing process, including the amount and purpose of each substance. In most PIs, this information is found -

Potassium Phosphate (Dibasic)
G-Biosciences, St Louis, MO, USA | 1-800-628-7730 | 1-314-991-6034 | [email protected] A Geno Technology, Inc. (USA) brand name Safety Data Sheet Cat. # RC-082 Potassium Phosphate (Dibasic) Size: 1kg think proteins! think G-Biosciences! www.GBiosciences.com Potassium Phosphate (Dibasic) Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH) with its amendment Regulation (EU) 2015/830 Revision date: 5/11/2017 Version: 1.1 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product form : Substance Substance name : Potassium Phosphate (Dibasic) Chemical name : Potassium Phosphate (Dibasic) EC-No. : 231-834-5 CAS-No. : 7758-11-4 Product code : 316P Type of product : Pure substance,Hygroscopic substance. Preventive measures apply to the substance in dry state only Formula : K2HPO4 Synonyms : dibasic potassium phosphate, anhydrous / dikalium phosphate, anhydrous / dipotassium hydrogen phosphate, anhydrous / dipotassium hydrogenorthophosphate, anhydrous / dipotassium monohydrogen phosphate, anhydrous / dipotassium monophosphate, anhydrous / dipotassium orthophosphate, anhydrous / dipotassium phosphate, anhydrous / dipotassium-o-phosphate, anhydrous / DKP, anhydrous / hydrogen dipotassium phosphate, anhydrous / orthophosphate dipotassium, anhydrous / phosphoric acid, dipotassium salt / phosphoric acid, dipotassium salt, anhydrous / potassium biphosphate, anhydrous / potassium dibasic phosphate, anhydrous / potassium hydrogen phosphate(=di potassium hydrogen phosphate) -

General Properties of the Alkaline Phosphates: - Major Food and Technical Applications
Phosphorus Research Bulletin Vol. 15 (2004) p. 85-94 General Properties of the Alkaline Phosphates: - Major Food and Technical Applications P.HOURANT Deputy Business Line Manager, Prayon S.A., Business Unit Phosphates, Rue Joseph Wauters, 144 4480 Engis, Belgium; E-mail: [email protected] INTRODUCTION The alkaline phosphates are used for many food and technical applications. Phosphates have two characteristics that explain their four main properties: buffer agent, sequestering power, dispersing power and water holding capability. Those properties allow phosphates to be used in many food and technical applications. The main food applications are meat and seafood processing, baking and processed cheese, but others such as cereals, French fries, fruits and vegetables, beverages, noodles and so on also may need the use of phosphates. On the technical side, the main applications are the detergent products, the water treatment and the metal treatment. As for the food, many other applications require phosphates such as ceramics, bone china, paper and paints,... In meat products, phosphates salts interact in a unique way to bind water with proteins and improve the tenderness in meats. Treated products will maintain their juicy appearance as well as their natural nutritional properties texture and colour. In fish and seafood products, phosphates salts allow the retention of the natural juices of frozen fish fillets, prawns, shrimps, scallops and other seafood. Phosphates also help prevent the build-up of struvite crystals in tinned tuna and crabmeat. In processed cheese, phosphates are crucially important in the production of processed cheese. These products ensure a homogeneous and uniform melt of raw cheese and product stability. -

IFAC Summary of Phosphate Citations the International Food Additives
IFAC Summary of Phosphate Citations The International Food Additives Council (IFAC) is a global association representing manufacturers of food ingredients, including phosphates used as food additives. IFAC strives for the harmonization of food additive standards and specifications worldwide, and supports regulatory processes to identify, categorize and document the safety of food additives. Phosphorus is an essential element critical for several key biochemical processes in the body, including development of cell membranes, growth of bones and teeth, maintenance of acid-base balance, and cellular energetics. Phosphorus is naturally occurring in various types of foods, including meat, grains, and dairy. Additionally, inorganic phosphates can be added to foods to improve texture, flavor, shelf life, and other technological functions. Inorganic phosphates are salts or esters of phosphoric acid. Phosphoric acid is produced starting with naturally-occurring phosphate ore mined around the world. As phosphoric acid, it can be combined with other elements such as calcium, potassium, and sodium into "salts." Phosphate additives are contained in a large number of processed foods and beverages and help contribute to the vast food supply while also minimizing food waste. Following is a comprehensive list of phosphates that are approved for use in food. All of these phosphates have either been approved by the US Food and Drug Administration (FDA) as a direct food additive or reviewed by FDA and determined to be generally recognized as safe (GRAS). Also included are the CAS numbers, International Numbering System (INS) numbers, Food Chemicals Codex (FCC) references and Joint FAO/WHO Expert Committee on Food Additives (JECFA) evaluations, as available. -
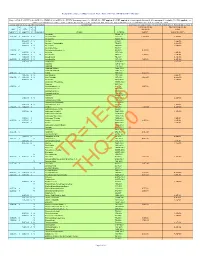
Composite Worker Air RSL May 2021 HQ10
Regional Screening Level (RSL) Composite Worker Ambient Air Table (TR=1E-06, HQ=1) May 2021 Key: I = IRIS; P = PPRTV; O = OPP; A = ATSDR; C = Cal EPA; X = PPRTV Screening Level; H = HEAST; W = TEF applied; E = RPF applied; G = user's guide Section 5; M = mutagen; V = volatile; R = RBA applied ; c = cancer; n = noncancer; * = where: n SL < 100X c SL; ** = where n SL < 10X c SL; SSL values are based on DAF=1; m = ceiling limit exceeded; s = Csat exceeded. Toxicity and Chemical-specific Information Contaminant Carcinogenic Target Risk (TR) = 1E-06 Noncancer Hazard Index (HI) = 1 k k v Carcinogenic SL Noncarcinogenic SL IUR e RfCi e o TR=1E-06 THI=1 (ug/m3)-1 y (mg/m3) y l mutagen Analyte CAS No. (ug/m3) (ug or fibers/m3) Acephate 30560-19-1 2.2E-06 I 9.0E-03 I V Acetaldehyde 75-07-0 5.6E+00 3.9E+01 Acetochlor 34256-82-1 3.1E+01 A V Acetone 67-64-1 1.4E+05 2.0E-03 X Acetone Cyanohydrin 75-86-5 8.8E+00 6.0E-02 I V Acetonitrile 75-05-8 2.6E+02 V Acetophenone 98-86-2 1.3E-03 C Acetylaminofluorene, 2- 53-96-3 9.4E-03 2.0E-05 I V Acrolein 107-02-8 8.8E-02 1.0E-04 I 6.0E-03 I M Acrylamide 79-06-1 1.2E-01 2.6E+01 1.0E-03 I V Acrylic Acid 79-10-7 4.4E+00 6.8E-05 I 2.0E-03 I V Acrylonitrile 107-13-1 1.8E-01 8.8E+00 6.0E-03 P Adiponitrile 111-69-3 2.6E+01 Alachlor 15972-60-8 Aldicarb 116-06-3 Aldicarb Sulfone 1646-88-4 Aldicarb sulfoxide 1646-87-3 4.9E-03 I V Aldrin 309-00-2 2.5E-03 1.0E-04 X V Allyl Alcohol 107-18-6 4.4E-01 6.0E-06 C 1.0E-03 I V Allyl Chloride 107-05-1 2.0E+00 4.4E+00 5.0E-03 P Aluminum 7429-90-5 2.2E+01 Aluminum Phosphide 20859-73-8 -

WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C12N 15/10 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/DK20 15/050343 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 11 November 2015 ( 11. 1 1.2015) KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (25) Filing Language: English PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (26) Publication Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: PA 2014 00655 11 November 2014 ( 11. 1 1.2014) DK (84) Designated States (unless otherwise indicated, for every 62/077,933 11 November 2014 ( 11. 11.2014) US kind of regional protection available): ARIPO (BW, GH, 62/202,3 18 7 August 2015 (07.08.2015) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicant: LUNDORF PEDERSEN MATERIALS APS TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, [DK/DK]; Nordvej 16 B, Himmelev, DK-4000 Roskilde DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, (DK). -

Redox Pty Ltd 2 Swettenham Road +61-2-97333000 Minto NSW 2566 Australia
Safety Data Sheet Dipotassium phosphate (DKP) Revision 4, Date 20 Jun 2018 1. IDENTIFICATION Product Name Dipotassium phosphate (DKP) Other Names Dipotassium hydrogen phosphate; Potassium phosphate, dibasic Uses Fertiliser, food additive, buffering agent. Chemical Family No Data Available Chemical Formula K2HPO4 Chemical Name Phosphoric acid, dipotassium salt Product Description No Data Available Contact Details of the Supplier of this Safety Data Sheet Organisation Location Telephone Redox Pty Ltd 2 Swettenham Road +61-2-97333000 Minto NSW 2566 Australia Redox Pty Ltd 11 Mayo Road +64-9-2506222 Wiri Auckland 2104 New Zealand Redox Inc. 3960 Paramount Boulevard +1-424-675-3200 Suite 107 Lakewood CA 90712 USA Redox Chemicals Sdn Bhd Level 2, No. 8, Jalan Sapir 33/7 +60-3-5614-2111 Seksyen 33, Shah Alam Premier Industrial Park 40400 Shah Alam Sengalor, Malaysia Emergency Contact Details For emergencies only; DO NOT contact these companies for general product advice. Organisation Location Telephone Poisons Information Centre Westmead NSW 1800-251525 131126 Chemcall Australia 1800-127406 +64-4-9179888 Chemcall Malaysia +64-4-9179888 Chemcall New Zealand 0800-243622 +64-4-9179888 National Poisons Centre New Zealand 0800-764766 CHEMTREC USA & Canada 1-800-424-9300 CN723420 +1-703-527-3887 2. HAZARD IDENTIFICATION Poisons Schedule (Aust) Not Scheduled Globally Harmonised System Redox Pty Ltd Australia New Zealand Malaysia Corporate Office Sydney Phone +61 2 9733 3000 Adelaide Auckland Kuala Lumpur Locked Bag 15 Minto NSW 2566 Australia Fax +61 -
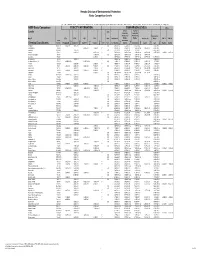
Basic Comparison Levels
Nevada Division of Environmental Protection Basic Comparison Levels Key: I=IRIS; P= PPRTV; N=NCEA; H=HEAST; A=ATSDR; O=Other Documents; CA=CalEPA S=Surrogate X=Appendix PPRTV E=Based on TEF scheme r=Route Extra Key: C = Cancer endpoint; N = Noncancer endpoint; sat = Saturation Limit; max = Ceiling Limit NDEP Basic Comparison TOXICITY INFORMATION COMPARISON LEVELS LBCLs Indoor Outdoor Levels Skin Industrial/ Industrial/ Commercial Commercial Residential May-17 SFo RfDo IUR RfCi Abs. Residential Worker Worker Ambient Air Water DAF 1 DAF 20 CAS w/o Dermal Chemical Constituents Number 1/(mg/kg-d) (mg/kg-d) (ug/m3)-1 (mg/m3) VOCc Soils Soil (mg/kg) (mg/kg) Soil (mg/kg) (µg/m3) (µg/l) (mg/kg) (mg/kg) Key Key key Key Key Key Key Key Key Acephate 30560-19-1 8.70E-03 I 4.00E-03 I 0.10 5.59E+01 C 7.52E+02 C 2.95E+02 C 7.73E+00 C Acetaldehyde 75-07-0 2.20E-06 I 9.00E-03 I V 1.23E+01 C 5.35E+01 C 1.00E+05 max 1.28E+00 C 2.55E+00 C Acetochlor 34256-82-1 2.00E-02 I 0.10 1.23E+03 N 4.67E+04 N 1.83E+04 N 6.67E+02 N Acetone 67-64-1 9.00E-01 I 3.10E+01 A V 7.04E+04 N 1.00E+05 max 1.00E+05 max 3.23E+04 N 2.05E+04 N 8.00E-01 1.60E+01 Acetone Cyanohydrin 75-86-5 2.00E-03 X 0.10 1.00E+05 max 1.00E+05 max 1.00E+05 max 2.09E+00 N Acetonitrile 75-05-8 6.00E-02 I V 1.00E+05 max 3.75E+03 N 1.00E+05 max 6.26E+01 N 1.25E+02 N Acetophenone 98-86-2 1.00E-01 I V 2.52E+03 sat 2.52E+03 sat 2.52E+03 sat 3.34E+03 N Acetylaminofluorene, 2- 53-96-3 3.80E+00 CA 1.30E-03 CA 0.10 1.28E-01 C 1.72E+00 C 6.75E-01 C 2.16E-03 C 1.77E-02 C Acrolein 107-02-8 5.00E-04 I 2.00E-05 I V -

( 12 ) United States Patent
US010212933B2 (12 ) United States Patent (10 ) Patent No. : US 10 ,212 , 933 B2 Hemminghaus et al. ( 45 ) Date of Patent : * Feb . 26 , 2019 ( 54 ) LOW VOLATILITY HERBICIDAL 8 ,987 , 167 B2 3 / 2015 Xu et al. 2002 /0108415 A1 8 / 2002 Volgas et al . COMPOSITIONS 2002 /0160916 Al 10 / 2002 Volgas et al. 2006 /0270557 A1 11 /2006 Volgas et al . (71 ) Applicant : Monsanto Technology LLC , St. Louis , 2010 /0248963 AL 9 /2010 Becher et al. MO (US ) 2010 / 0331182 Al 12 /2010 Zhang et al. 2011 / 0009269 Al 1 / 2011 Gioia et al. (72 ) Inventors : John W . Hemminghaus, St. Louis , MO 2011 /0034332 A1 2 / 2011 Becher et al. 2011/ 0166235 A1 7 / 2011 Sun (US ) ; Alison MacInnes , St . Louis , MO 2012 / 0142532 Al 6 / 2012 Wright et al . (US ) ; Daniel R . Wright, St. Louis , MO 2012 /0231956 A1 9 / 2012 Rainbird (US ) ; Junhua Zhang , St. Louis , MO 2012 / 0238451 Al 9 /2012 Feng et al . (US ) 2012 / 0289402 A1 11/ 2012 Brown et al . 2013 /0079228 A1 3 / 2013 Freed Subject to any disclaimer, the term of this 2013 /0109572 A1 5 / 2013 Pernak et al . ( * ) Notice : 2013 /0109725 A1 5 / 2013 Dave et al . patent is extended or adjusted under 35 2013 / 0225405 Al 8 / 2013 Hixson et al . U . S . C . 154 (b ) by 0 days . 2014 / 0013654 A1 1 / 2014 Burke This patent is subject to a terminal dis 2015 / 0057157 Al 2 / 2015 Baseeth et al . claimer . FOREIGN PATENT DOCUMENTS (21 ) Appl. No .: 15 /653 , 772 CN 1176954 A 3 / 1998 CN 101564044 A 10 / 2009 (22 ) Filed : Jul.