Page: Treatment-Drugs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
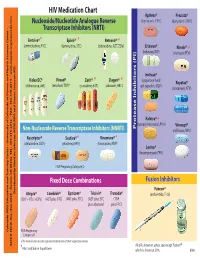
HIV Medication Chart Aptivus® Prezista® Nucleoside/Nucleotide Analogue Reverse (Tipranavir, TPV) (Darunavir, DRV) Transcriptase Inhibitors (NRTI)
HIV Medication Chart Aptivus® Prezista® Nucleoside/Nucleotide Analogue Reverse (tipranavir, TPV) (darunavir, DRV) Transcriptase Inhibitors (NRTI) Emtriva®* Epivir® * Retrovir® * (emtricitabine, FTC) (lamivudine, 3TC) (zidovudine, AZT, ZDV) Crixivan® Norvir® * (indinavir, IDV) (ritonavir, RTV) Invirase® Videx EC® Viread® Zerit® Ziagen® * * (saquinavir hard Reyataz® (didanosine, ddl) (tenofovir,TDF)* (stavudine, d4T) (abacavir, ABC) gel capsules, SQV) (atazanavir, ATV) Kaletra® * (lopinavir/ritonavir, LPV/r) Viracept® Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTI) Inhibitors (PI) Protease (nelfinavir, NFV) Rescriptor® Sustiva®* Viramune® * (delavirdine, DLV) (efavirenz, EFV) (nevirapine, NVP) Lexiva® (fosamprenavir, FPV) FDA Pregnancy Category D Fixed Dose Combinations Fusion Inhibitors Fuzeon® Developed by MeriLou Johnson, MeriLou by Developed Johnson, MSW, and Steven MPA MD Atripla® Combivir® Epzicom® Trizivir® Truvada® (enfuvirtide,T-20) (TDF+FTC+EFV) (AZT plus 3TC) (ABC plus 3TC) (AZT plus 3TC (TDF plus abacavir) plus FTC) Colorado AIDS Education and Training Center • University of Colorado at Denver and Health Sciences Center • Center and Health Sciences Denver at of Colorado • University Center Training and AIDS Education Colorado FDA Pregnancy Category D ®The brands listed are the registered trademarks of their respective owners. 4200 E. Ave., Ninth TEL: A089 • Denver, Box 80262 CO 303-315-2512 • FAX: • 303-315-2514 • www.mpaetc.org/colorado.htm All pills shown in actual size except Fuzeon® *Also available in liquid form. which is shown at 50%. 8/06 Medication Schedule Name______________________________ Date___________ Number of Time of day you ar to take TOTAL Name of Medication pills to take this medicine Food Interactions Side Effects number of each time pills each day With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ Discontinued Medications or Formulations Helpful Hints: • Refill prescriptions before you run out. -

Truvada (Emtricitabine / Tenofovir Disoproxil)
Pre-exposure Prophylaxis (2.3) HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Recommended dose in HIV-1 uninfected adults: One tablet TRUVADA safely and effectively. See full prescribing information (containing 200 mg/300 mg of emtricitabine and tenofovir for TRUVADA. disoproxil fumarate) once daily taken orally with or without food. (2.3) TRUVADA® (emtricitabine/tenofovir disoproxil fumarate) tablets, for oral use Recommended dose in renally impaired HIV-uninfected Initial U.S. Approval: 2004 individuals: Do not use TRUVADA in HIV-uninfected individuals if CrCl is below 60 mL/min. If a decrease in CrCl is observed in WARNING: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH uninfected individuals while using TRUVADA for PrEP, evaluate STEATOSIS, POST-TREATMENT ACUTE EXACERBATION OF potential causes and re-assess potential risks and benefits of HEPATITIS B, and RISK OF DRUG RESISTANCE WITH USE OF continued use. (2.4) TRUVADA FOR PrEP IN UNDIAGNOSED HIV-1 INFECTION -----------------------DOSAGE FORMS AND STRENGTHS------------------- See full prescribing information for complete boxed warning. Tablets: 200 mg/300 mg, 167 mg/250 mg, 133 mg/200 mg, and 100 Lactic acidosis and severe hepatomegaly with steatosis, mg/150 mg of emtricitabine and tenofovir disoproxil fumarate . (3) including fatal cases, have been reported with the use of nucleoside analogs, including VIREAD, a component of TRUVADA. (5.1) --------------------------------CONTRAINDICATIONS----------------------------- TRUVADA is not approved for the treatment of chronic Do not use TRUVADA for pre-exposure prophylaxis in individuals with hepatitis B virus (HBV) infection. Severe acute unknown or positive HIV-1 status. TRUVADA should be used in exacerbations of hepatitis B have been reported in patients HIV-infected patients only in combination with other antiretroviral coinfected with HIV-1 and HBV who have discontinued agents. -

Download Article PDF/Slides
New Antiretrovirals in Development: Reprinted from The PRN Notebook,™ june 2002. Dr. James F. Braun, Editor-in-Chief. Tim Horn, Executive Editor. Published in New York City by the Physicians’ Research Network, Inc.,® John Graham Brown, Executive Director. For further information and other articles The View in 2002 available online, visit http://www.PRN.org All rights reserved. © june 2002. Roy “Trip” Gulick, md, mph Associate Professor of Medicine, Weill Medical College of Cornell University Director, Cornell Clinical Trials Unit, New York, New York Summary by Tim Horn Edited by Scott Hammer, md espite the fact that 16 antiretro- tiviral activity of emtricitabine was estab- Preliminary results from two random- virals are approved for use in the lished, with total daily doses of 200 mg or ized studies—FTC-302 and FTC-303—were United States, there is an indis- more producing the greatest median viral reported by Dr. Charles van der Horst and putable need for new anti-hiv com- load suppression: 1.72-1.92 log. Based on his colleagues at the 8th croi, held in Feb- pounds that have potent and these data, a once-daily dose of 200 mg ruary 2001 in Chicago (van der Horst, durable efficacy profiles, unique re- was selected for further long-term clinical 2001). FTC-302 was a blinded comparison sistance patterns, patient-friendly dosing study. “This is what we’re looking forward of emtricitabine and lamivudine, both in schedules, and minimal toxicities. To pro- to with emtricitabine,” commented Dr. combination with stavudine (Zerit) and vide prn with a glimpse of drugs current- Gulick. -

Selected Properties of Maraviroc
Selected Properties of Maraviroc Other names UK-427,857, MVC, Celsentri , Selzentry (US) Manufacturer ViiV Healthcare ULC Pharmacology/Mechanism of Maraviroc is a selective, slowly reversible, small molecule Action antagonist of the interaction between human CCR5 and HIV-1 gp120. Blocking this interaction prevents CCR5-tropic HIV-1 entry into cells . CCR5 antagonists target a discrete step in the viral entry pathway. The mechanism of HIV entry into the host CD4 T cells involves a sequence of molecular interactions between the virion envelope glycoprotein (Env) and host cell surface receptors. Normally, the gp120 Env subunit binds to CD4, and subsequent binding of HIV to the host cell’s coreceptors (CCR5 or CXCR4) causes a conformational change leading to membrane fusion into the host cell. Allosteric binding of a CCR5 antagonist results in a receptor conformation that the virus cannot bind to, thus interfering with the fusion process. NB: Use of maraviroc is not recommended in patients with dual/mixed or CXCR4-tropic HIV-1 as efficacy was not demonstrated in a phase 2 study of this patient group. The mean EC value (50% effective concentration) for Activity 50 maraviroc against HIV-1 group M isolates (clades A to J) and group O isolates ranged from 0.1 to 1.25 nM (0.05 to 0.64 ng/mL) in cell culture. Mean potency against a range of CCR5- tropic clinical primary isolates: IC 90 2.03 nM (1.04 ng/mL). In 973 treatment-experienced HIV-1-infected subjects in studies A4001027 and A4001028, the C , baseline viral load, baseline min CD4, cell count and overall sensitivity score (OSS) were found to be important predictors of virologic success (defined as viral load < 400 copies/mL at 24 weeks). -

(12) Patent Application Publication (10) Pub. No.: US 2008/0241135 A1 Olson Et Al
US 20080241135A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0241135 A1 Olson et al. (43) Pub. Date: Oct. 2, 2008 (54) METHODS FOR REDUCINGVIRAL LOAD IN 60/711,528, filed on Aug. 26, 2005, provisional appli HIV-1-INFECTED PATIENTS cation No. 60/715,619, filed on Sep. 9, 2005. (75) Inventors: William C. Olson, Ossining, NY Publication Classification (US); Paul J. Maddon, Scarsdale, NY (US); Daniel C. Pevear, (51) Int. Cl. Medford, MA (US); Robert J. A 6LX 39/395 (2006.01) Israel, Suffern, NY (US); Jose D. A6IP 43/00 (2006.01) Murga, Rosedale, NY (US) (52) U.S. Cl. ..................................................... 424/133.1 Correspondence Address: (57) ABSTRACT COOPER & DUNHAM, LLP 1185 AVENUE OF THE AMERICAS This method provides a method for reducing HIV-1 viral load NEW YORK, NY 10036 in an HIV-1-infected human subject which comprises admin istering to the subject at a predefined interval effective HIV-1 (73) Assignee: Progenics Pharmaceuticals, Inc. viral load-reducing doses of (a) a humanized antibody desig nated PRO 140, or of (b) an anti-CCR5 receptor monoclonal (21) Appl. No.: 11/894,762 antibody. This invention also provides a method for inhibiting in a human Subject the onset or progression of an HIV-1- (22) Filed: Aug. 20, 2007 associated disorder, the inhibition of which is effected by inhibiting fusion of HIV-1 to CCR5"CD4" target cells in the Related U.S. Application Data Subject. This invention also provides a method for treating a subject infected with HIV-1 comprising administering to the (63) Continuation of application No. -

SELZENTRY (Maraviroc) Tablets, for Oral Use • Hepatotoxicity Accompanied by Severe Rash Or Systemic Allergic Reaction
---------------------------------- CONTRAINDICATIONS --------------------------------- HIGHLIGHTS OF PRESCRIBING INFORMATION • SELZENTRY is contraindicated in patients with severe renal impairment These highlights do not include all the information needed to use or end-stage renal disease (ESRD) (CrCl less than 30 mL per minute) SELZENTRY safely and effectively. See full prescribing information for who are concomitantly taking potent CYP3A inhibitors or inducers. (4) SELZENTRY. -------------------------- WARNINGS AND PRECAUTIONS -------------------------- SELZENTRY (maraviroc) tablets, for oral use • Hepatotoxicity accompanied by severe rash or systemic allergic reaction, SELZENTRY (maraviroc) oral solution including potentially life-threatening events, has been reported. Hepatic Initial U.S. Approval: 2007 laboratory parameters including ALT, AST, and bilirubin should be obtained prior to starting SELZENTRY and at other time points during WARNING: HEPATOTOXICITY See full prescribing information for complete boxed warning. treatment as clinically indicated. If rash or symptoms or signs of hepatitis or allergic reaction develop, hepatic laboratory parameters should be • Hepatotoxicity has been reported which may be preceded by severe monitored and discontinuation of treatment should be considered. When rash or other features of a systemic allergic reaction (e.g., fever, administering SELZENTRY to patients with pre-existing liver eosinophilia, or elevated IgE). (5.1) dysfunction or who are co-infected with hepatitis B and/or C virus, • -

SYMTUZA™ (Darunavir, Cobicistat, Emtricitabine, Tenofovir Alafenamide) Oral
PHARMACY COVERAGE GUIDELINES ORIGINAL EFFECTIVE DATE: 9/20/2018 SECTION: DRUGS LAST REVIEW DATE: 8/19/2021 LAST CRITERIA REVISION DATE: 8/19/2021 ARCHIVE DATE: SYMTUZA™ (darunavir, cobicistat, emtricitabine, tenofovir alafenamide) oral Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy Coverage Guideline must be read in its entirety to determine coverage eligibility, if any. This Pharmacy Coverage Guideline provides information related to coverage determinations only and does not imply that a service or treatment is clinically appropriate or inappropriate. The provider and the member are responsible for all decisions regarding the appropriateness of care. Providers should provide BCBSAZ complete medical rationale when requesting any exceptions to these guidelines. The section identified as “Description” defines or describes a service, procedure, medical device or drug and is in no way intended as a statement of medical necessity and/or coverage. The section identified as “Criteria” defines criteria to determine whether a service, procedure, medical device or drug is considered medically necessary or experimental or investigational. State or federal mandates, e.g., FEP program, may dictate that any drug, device or biological product approved by the U.S. Food and Drug Administration (FDA) may not be considered experimental or investigational and thus the drug, device or biological product may be assessed only on the basis of medical necessity. Pharmacy Coverage Guidelines are subject to change as new information becomes available. For purposes of this Pharmacy Coverage Guideline, the terms "experimental" and "investigational" are considered to be interchangeable. -

Invirase, INN-Saquinavir
SCIENTIFIC DISCUSSION This module reflects the initial scientific discussion for the approval of Invirase. This scientific discussion has been updated until 1 October 2004. For information on changes after this date please refer to module 8B 1. Chemical, pharmaceutical and biological aspects Composition Capsules are supplied in amber glass bottle with a polypropylene screw closure, PVC sealing gasket and a polyurethane pad. No desiccant unit is included in the marketed pack. Active substance Saquinavir possesses six chiral centres resulting in 64 possible stereoisomers but only one stereoisomer is selected for the formulation intended for marketing. The synthesis of the active substance consists of a multi steps process. Following further development during the post-marketing phase, the synthesis has however been simplified to improve the efficiency of the process. The correct stereoisomeric form of saquinavir is dependent on the quality and chiral purity of the key intermediates used during the synthesis. The impurities arising from synthesis have been well specified. Product development and finished medicinal product Saquinavir is characterised by low aqueous solubility, low oral bioavailability (approximately 4 %) and bitter taste. The development of the formulation intended for marketing is adequate to deal with these characteristics. Invirase is presented in a hard gelatine capsule containing micronised saquinavir mesylate and standard excipients. Key steps of the manufacturing process (granulation, drying, batch homogeneity and capsule filling) are adequately described and validated and results of batch analysis demonstrate the consistency of the manufacturing process. Capsules are tested according to standard methods. Analytical methods are in general well described and validated. The dissolution limit for the capsules equivalent to 75 % after 45 minutes as proposed by the applicant was considered a tight limit. -

Treatment of AIDS and HIV-Related Conditions: 2001 J Am Board Fam Pract: First Published As on 1 July 2001
Current Report—HIV Treatment of AIDS and HIV-Related Conditions: 2001 J Am Board Fam Pract: first published as on 1 July 2001. Downloaded from Ronald H. Goldschmidt, MD, and Betty J. Dong, PharmD Managing human immunodeficiency virus (HIV) offer the best opportunity to provide comprehen- disease and the acquired immunodeficiency syn- sive care. drome (AIDS) has become more standardized yet This Current Report—HIV updates our annual more complex during the past year. Antiretroviral treatment guidelines.3 These recommendations treatment guidelines now represent a general con- (Table 1) are based on our experience at San Fran- sensus on basic treatment principles and options1 as cisco General Hospital, published guidelines, a re- benefits and risks of therapy have become more view of the medical literature, and experience evident. Clinical manifestations of HIV infection, gained from answering telephone calls to our Na- prognosis, and quality of life are clearly improved tional HIV Telephone Consultation Service for most patients receiving potent antiretroviral (Warmline). Because HIV management changes therapy, yet viral resistance and chronic and short- rapidly, clinicians are advised to refer to the excel- term drug toxicities remain major problems. The lent federal guidelines for the use of antiretroviral markedly decreased incidence of opportunistic in- agents in HIV-infected adults and adolescents1 and fections among treated patients has made unusual for prevention of opportunistic infections,4 which infections less common in daily HIV management. are updated frequently on the Internet (Table 2). The role of resistance assays in HIV care is still These and other federal guidelines are available at being assessed, but they offer great possibilities for http://www.hivatis.org. -

(Lupin Limited), HA659 WHOPAR Part 4 US Prescribing Information March 2016 Page 1 of 23
Lamivudine/Zidovudine 150mg/300mg WHOPAR part 4 March 2016 Tablets US prescribing information (Lupin Limited), HA659 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ---------------------- WARNINGS AND PRECAUTIONS ---------------------- . lamivudine and zidovudine tablets safely and effectively. See full See boxed warning for information about the following: hematologic toxicity, symptomatic myopathy, lactic acidosis and severe prescribing information for lamivudine and zidovudine tablets. hepatomegaly, and severe acute exacerbations of hepatitis B. (5.1, 5.2, Lamivudine and Zidovudine Tablets USP, 150 mg/300 mg 5.3, 5.4) . Lamivudine and zidovudine tablets should not be administered with Initial U.S. Approval: 1997 other lamivudine- or zidovudine-containing products or emtricitabine- containing products. (5.5) WARNING: RISK OF HEMATOLOGIC TOXICITY, MYOPATHY, . Hepatic decompensation, some fatal, has occurred in HIV-1/HCV co- LACTIC ACIDOSIS, EXACERBATIONS OF HEPATITIS B infected patients receiving combination antiretroviral therapy and See full prescribing information for complete boxed warning. interferon alfa with/without ribavirin. Discontinue lamivudine and • Hematologic toxicity including neutropenia and anemia have been zidovudine tablets as medically appropriate and consider dose reduction associated with the use of zidovudine, one of the components of or discontinuation of interferon alfa, ribavirin, or both. (5.6) lamivudine and zidovudine tablets. (5.1) . Exacerbation of anemia has been reported in HIV-1/HCV co-infected • Symptomatic myopathy associated with prolonged use of zidovudine. patients receiving ribavirin and zidovudine. Co-administration of (5.2) ribavirin and zidovudine is not advised. (5.6) • Lactic acidosis and hepatomegaly with steatosis, including fatal cases, . Pancreatitis: Use with caution in pediatric patients with a history of have been reported with the use of nucleoside analogues including pancreatitis or other significant risk factors for pancreatitis. -

The HIV Protease Inhibitors Nelfinavir and Saquinavir, but Not a Variety Of
Antiviral Therapy 10:215–223 The HIV protease inhibitors nelfinavir and saquinavir, but not a variety of HIV reverse transcriptase inhibitors, adversely affect human proteasome function Marco Piccinini1, Maria T Rinaudo1*, Annalisa Anselmino1, Barbara Buccinnà1, Cristina Ramondetti1, Antonio Dematteis1, Emanuela Ricotti 2, Lucia Palmisano3, Michael Mostert2 and Pier-Angelo Tovo2 1Department of Medicine and Experimental Oncology and 2Department of Paediatric Sciences, University of Turin, Turin, Italy 3Istituto Superiore di Sanità, Rome, Italy *Corresponding author: Tel: +39 011 670 5303 Fax: +39 011 670 5311; E-mail: [email protected] Background: In HIV-infected patients some clinical and drugs at different concentrations. Intracellular protea- immunological benefits of antiretroviral therapy, which some proteolytic activity was evaluated by searching for frequently include a combination of HIV protease inhibitors ubiquitin-tagged proteins in HL60 cells incubated with (PIs) and reverse transcriptase inhibitors (RTIs), cannot be and without the drugs. solely explained by the drugs’ action on viral enzymes. Results: At therapeutic dosages, nelfinavir and saquinavir Proteasomes constitute the central protease of the ubiq- inhibited proteasome peptidase activity and caused uitin ATP-dependent pathway involved in many cellular intracellular accumulation of polyubiquitinated proteins, processes, as well as in HIV maturation and aggressiveness. a hallmark of proteasome proteolytic inhibition in vivo; Objective: To explore whether the PIs nelfinavir and the RTIs failed to evoke either effect. saquinavir and the RTIs abacavir, nevirapine, delavirdine, Conclusion: Proteasomes are targeted by the two PIs but stavudine and didanosine affect proteasome function in not the RTIs. Therefore, in HIV-infected patients the vitro and in vivo. beneficial effect of a therapy including one of the two Methods: Peptidase activity of purified human 26S and PIs should partly rely on inhibition of host proteasome 20S proteasomes was assayed with and without the function. -

Association Among Ccr5 Genotypes, Ccr5 Expression, and In
ASSOCIATION AMONG CCR5 GENOTYPES, CCR5 EXPRESSION, AND IN VITRO HIV INFECTION Bangan John Submitted in partial fulfilment of the requirements For the degree of Master of Science Dissertation Advisor: Dr Peter A. Zimmerman Department of Biology CASE WESTERN RESERVE UNIVERSITY May, 2013 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the thesis/dissertation of Bangan John ______________________________________________________ Master of Science candidate for the ________________________________degree *. Roy Ritzmann (signed)_______________________________________________ (chair of the committee) Peter Zimmerman ________________________________________________ Christopher Cullis ________________________________________________ Daniel Tisch ________________________________________________ 12/20/2012 (date) _______________________ *We also certify that written approval has been obtained for any proprietary material contained therein. Dedication: I would like to dedicate this thesis to my family, the John’s. Table of Contents List of Tables ................................................................................................................. ii List of Figures .............................................................................................................. iii Acknowledgements ....................................................................................................... iv Abbreviations ................................................................................................................