Drug Class Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Treatment-Drugs
© National HIV Curriculum PDF created September 23, 2021, 9:14 am Stavudine (Zerit) Table of Contents Stavudine Zerit Summary Drug Summary Key Clinical Trials Resistance Key Drug Interactions Drug Summary Stavudine, an early nucleoside reverse transcriptase inhibitor (NRTI), was used as part of combination antiretroviral therapy for years, but now has become obsolete and replaced by better-tolerated and safer options. Stavudine poses risk of serious toxicity, including peripheral neuropathy (which can be permanent), pancreatitis, lipoatrophy, and lactic acidosis. Fatal and nonfatal cases of pancreatitis and lactic acidosis have been reported, especially when stavudine was combined with didanosine. According to the Adult and Adolescent ARV Guidelines, stavudine is no longer recommended for the treatment of HIV infection due to potential severe toxicity. Further, all persons currently taking stavudine should be strongly encouraged to switch to a safer medication. The sale and distribution of all strengths of stavudine will be discontinued and removed from the market in the United States in 2020. Key Clinical Trials Stavudine was studied for treatment-naïve patients as part of triple therapy, such as with lamivudine plus indinavir [START I], lamivudine plus lopinavir-ritonavir [M98-863], and lamivudine plus efavirenz [DART II]. Several studies demonstrated benefits of switching stavudine to newer NRTI agents, such as tenofovir disoproxil fumarate; the switch led to decreased rates of metabolic complications and mitochondrial toxicity [903E, SNAP, and ACTG 5142]. Resistance For a listing of the most common clinically significant mutations associated with stavudine (d4T) resistance, see the NRTI Resistance Notes on the Stanford University HIV Drug Resistance Database. Page 1/2 Key Drug Interactions For complete information on stavudine-related drug interactions, see the Drug Interactions section in the Stavudine (Zerit) Prescribing Information. -

Highlights of Prescribing Information
HIGHLIGHTS OF PRESCRIBING INFORMATION --------------------------WARNINGS AND PRECAUTIONS-------------------- These highlights do not include all the information needed to use • New onset or worsening renal impairment: Can include acute VIREAD safely and effectively. See full prescribing information renal failure and Fanconi syndrome. Assess creatinine clearance for VIREAD. (CrCl) before initiating treatment with VIREAD. Monitor CrCl and ® serum phosphorus in patients at risk. Avoid administering VIREAD (tenofovir disoproxil fumarate) tablets, for oral use VIREAD with concurrent or recent use of nephrotoxic drugs. (5.3) VIREAD® (tenofovir disoproxil fumarate) powder, for oral use • Coadministration with Other Products: Do not use with other Initial U.S. Approval: 2001 tenofovir-containing products (e.g., ATRIPLA, COMPLERA, and TRUVADA). Do not administer in combination with HEPSERA. WARNING: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH (5.4) STEATOSIS and POST TREATMENT EXACERBATION OF HEPATITIS • HIV testing: HIV antibody testing should be offered to all HBV- infected patients before initiating therapy with VIREAD. VIREAD See full prescribing information for complete boxed warning. should only be used as part of an appropriate antiretroviral • Lactic acidosis and severe hepatomegaly with steatosis, combination regimen in HIV-infected patients with or without HBV including fatal cases, have been reported with the use of coinfection. (5.5) nucleoside analogs, including VIREAD. (5.1) • Decreases in bone mineral density (BMD): Consider assessment • Severe acute exacerbations of hepatitis have been reported of BMD in patients with a history of pathologic fracture or other in HBV-infected patients who have discontinued anti- risk factors for osteoporosis or bone loss. (5.6) hepatitis B therapy, including VIREAD. Hepatic function • Redistribution/accumulation of body fat: Observed in HIV-infected should be monitored closely in these patients. -

Eparate Formulations According to the Prescribed Dosing Recommendations for These Products
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Lamivudine/Zidovudine Teva 150 mg/300 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 150 mg lamivudine and 300 mg zidovudine. For the full list of excipients see section 6.1. 3. PHARMACEUTICAL FORM Film-coated tablet White, capsule shaped, biconvex, film-coated scored tablet – engraved with “L/Z” on one side and “150/300” on the other side. The tablet can be divided into equal halves. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Lamivudine/Zidovudine Teva is indicated in antiretroviral combination therapy for the treatment of Human Immunodeficiency Virus (HIV) infection (see section 4.2). 4.2 Posology and method of administration Therapy should be initiated by a physician experienced in the management of HIV infection. Lamivudine/Zidovudine Teva may be administered with or without food. To ensure administration of the entire dose, the tablet(s) should ideally be swallowed without crushing. For patients who are unable to swallow tablets, tablets may be crushed and added to a small amount of semi-solid food or liquid, all of which should be consumed immediately (see section 5.2). Adults and adolescents weighing at least 30 kg: the recommended oral dose of Lamivudine/Zidovudine Teva is one tablet twice daily. Children weighing between 21 kg and 30 kg: the recommended oral dose of Lamivudine/Zidovudine Teva is one-half tablet taken in the morning and one whole tablet taken in the evening. Children weighing from 14 kg to 21 kg: the recommended oral dose of Lamivudine/Zidovudine Teva is one-half tablet taken twice daily. -

COVID-19) Pandemic on National Antimicrobial Consumption in Jordan
antibiotics Article An Assessment of the Impact of Coronavirus Disease (COVID-19) Pandemic on National Antimicrobial Consumption in Jordan Sayer Al-Azzam 1, Nizar Mahmoud Mhaidat 1, Hayaa A. Banat 2, Mohammad Alfaour 2, Dana Samih Ahmad 2, Arno Muller 3, Adi Al-Nuseirat 4 , Elizabeth A. Lattyak 5, Barbara R. Conway 6,7 and Mamoon A. Aldeyab 6,* 1 Clinical Pharmacy Department, Jordan University of Science and Technology, Irbid 22110, Jordan; [email protected] (S.A.-A.); [email protected] (N.M.M.) 2 Jordan Food and Drug Administration (JFDA), Amman 11181, Jordan; [email protected] (H.A.B.); [email protected] (M.A.); [email protected] (D.S.A.) 3 Antimicrobial Resistance Division, World Health Organization, Avenue Appia 20, 1211 Geneva, Switzerland; [email protected] 4 World Health Organization Regional Office for the Eastern Mediterranean, Cairo 11371, Egypt; [email protected] 5 Scientific Computing Associates Corp., River Forest, IL 60305, USA; [email protected] 6 Department of Pharmacy, School of Applied Sciences, University of Huddersfield, Huddersfield HD1 3DH, UK; [email protected] 7 Institute of Skin Integrity and Infection Prevention, University of Huddersfield, Huddersfield HD1 3DH, UK * Correspondence: [email protected] Citation: Al-Azzam, S.; Mhaidat, N.M.; Banat, H.A.; Alfaour, M.; Abstract: Coronavirus disease 2019 (COVID-19) has overlapping clinical characteristics with bacterial Ahmad, D.S.; Muller, A.; Al-Nuseirat, respiratory tract infection, leading to the prescription of potentially unnecessary antibiotics. This A.; Lattyak, E.A.; Conway, B.R.; study aimed at measuring changes and patterns of national antimicrobial use for one year preceding Aldeyab, M.A. -

Chapter 12 Antimicrobial Therapy Antibiotics
Chapter 12 Antimicrobial Therapy Topics: • Ideal drug - Antimicrobial Therapy - Selective Toxicity • Terminology - Survey of Antimicrobial Drug • Antibiotics - Microbial Drug Resistance - Drug and Host Interaction An ideal antimicrobic: Chemotherapy is the use of any chemical - soluble in body fluids, agent in the treatment of disease. - selectively toxic , - nonallergenic, A chemotherapeutic agent or drug is any - reasonable half life (maintained at a chemical agent used in medical practice. constant therapeutic concentration) An antibiotic agent is usually considered to - unlikely to elicit resistance, be a chemical substance made by a - has a long shelf life, microorganism that can inhibit the growth or - reasonably priced. kill microorganisms. There is no ideal antimicrobic An antimicrobic or antimicrobial agent is Selective Toxicity - Drugs that specifically target a chemical substance similar to an microbial processes, and not the human host’s. antibiotic, but may be synthetic. Antibiotics Spectrum of antibiotics and targets • Naturally occurring antimicrobials – Metabolic products of bacteria and fungi – Reduce competition for nutrients and space • Bacteria – Streptomyces, Bacillus, • Molds – Penicillium, Cephalosporium * * 1 The mechanism of action for different 5 General Mechanisms of Action for antimicrobial drug targets in bacterial cells Antibiotics - Inhibition of Cell Wall Synthesis - Disruption of Cell Membrane Function - Inhibition of Protein Synthesis - Inhibition of Nucleic Acid Synthesis - Anti-metabolic activity Antibiotics -

Epivir, INN-Lamivudine
SCIENTIFIC DISCUSSION This module reflects the initial scientific discussion for the approval of Epivir. This scientific discussion has been updated until 1 July 2005. For information on changes after this date please refer to module 8B. 1. Introduction The Human Immunodeficiency Virus (HIV) is a retrovirus, which replicates by transcribing its viral RNA genome into DNA via the enzyme reverse transcriptase (RT). The HIV results in a chronic, progressive deterioration in overall cell-mediated immunity with a steady depletion of CD4 T-lymphocytes causing Acquired Immunodeficiency Syndrome (AIDS). Agents of the family of 2’, -3’-dideoxynucleoside analogues are designed to suppress viral replication in two ways: by competitive inhibition with cellular nucleotides for the binding site of RT and, after incorporation into the growing viral DNA strand, by preventing further elongation of the viral DNA (chain termination). All the currently available nucleoside analogues have dose-limiting toxicities which may limit their long-term use. The development of resistant viruses in patients treated with these nucleosides analogues also demonstrates the pressing need for new agents to widen the choice of therapeutic agents available for treating patients infected with HIV. Lamivudine is a nucleoside analogue developed as a treatment for HIV infection. It has also activity against hepatitis B virus (HBV). It is metabolised intracellularly to the active moiety, lamivudine 5’- triphosphate. Its main mode of action is as a chain terminator of viral reverse transcription. The triphosphate has selective inhibitory activity against HIV-1 and HIV-2 replication in vitro, it is also active against zidovudine-resistant clinical isolates of HIV. -
HIV Medication Chart Aptivus® Prezista® Nucleoside/Nucleotide Analogue Reverse (Tipranavir, TPV) (Darunavir, DRV) Transcriptase Inhibitors (NRTI)
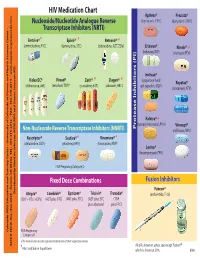
HIV Medication Chart Aptivus® Prezista® Nucleoside/Nucleotide Analogue Reverse (tipranavir, TPV) (darunavir, DRV) Transcriptase Inhibitors (NRTI) Emtriva®* Epivir® * Retrovir® * (emtricitabine, FTC) (lamivudine, 3TC) (zidovudine, AZT, ZDV) Crixivan® Norvir® * (indinavir, IDV) (ritonavir, RTV) Invirase® Videx EC® Viread® Zerit® Ziagen® * * (saquinavir hard Reyataz® (didanosine, ddl) (tenofovir,TDF)* (stavudine, d4T) (abacavir, ABC) gel capsules, SQV) (atazanavir, ATV) Kaletra® * (lopinavir/ritonavir, LPV/r) Viracept® Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTI) Inhibitors (PI) Protease (nelfinavir, NFV) Rescriptor® Sustiva®* Viramune® * (delavirdine, DLV) (efavirenz, EFV) (nevirapine, NVP) Lexiva® (fosamprenavir, FPV) FDA Pregnancy Category D Fixed Dose Combinations Fusion Inhibitors Fuzeon® Developed by MeriLou Johnson, MeriLou by Developed Johnson, MSW, and Steven MPA MD Atripla® Combivir® Epzicom® Trizivir® Truvada® (enfuvirtide,T-20) (TDF+FTC+EFV) (AZT plus 3TC) (ABC plus 3TC) (AZT plus 3TC (TDF plus abacavir) plus FTC) Colorado AIDS Education and Training Center • University of Colorado at Denver and Health Sciences Center • Center and Health Sciences Denver at of Colorado • University Center Training and AIDS Education Colorado FDA Pregnancy Category D ®The brands listed are the registered trademarks of their respective owners. 4200 E. Ave., Ninth TEL: A089 • Denver, Box 80262 CO 303-315-2512 • FAX: • 303-315-2514 • www.mpaetc.org/colorado.htm All pills shown in actual size except Fuzeon® *Also available in liquid form. which is shown at 50%. 8/06 Medication Schedule Name______________________________ Date___________ Number of Time of day you ar to take TOTAL Name of Medication pills to take this medicine Food Interactions Side Effects number of each time pills each day With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ Discontinued Medications or Formulations Helpful Hints: • Refill prescriptions before you run out. -

Truvada (Emtricitabine / Tenofovir Disoproxil)
Pre-exposure Prophylaxis (2.3) HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Recommended dose in HIV-1 uninfected adults: One tablet TRUVADA safely and effectively. See full prescribing information (containing 200 mg/300 mg of emtricitabine and tenofovir for TRUVADA. disoproxil fumarate) once daily taken orally with or without food. (2.3) TRUVADA® (emtricitabine/tenofovir disoproxil fumarate) tablets, for oral use Recommended dose in renally impaired HIV-uninfected Initial U.S. Approval: 2004 individuals: Do not use TRUVADA in HIV-uninfected individuals if CrCl is below 60 mL/min. If a decrease in CrCl is observed in WARNING: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH uninfected individuals while using TRUVADA for PrEP, evaluate STEATOSIS, POST-TREATMENT ACUTE EXACERBATION OF potential causes and re-assess potential risks and benefits of HEPATITIS B, and RISK OF DRUG RESISTANCE WITH USE OF continued use. (2.4) TRUVADA FOR PrEP IN UNDIAGNOSED HIV-1 INFECTION -----------------------DOSAGE FORMS AND STRENGTHS------------------- See full prescribing information for complete boxed warning. Tablets: 200 mg/300 mg, 167 mg/250 mg, 133 mg/200 mg, and 100 Lactic acidosis and severe hepatomegaly with steatosis, mg/150 mg of emtricitabine and tenofovir disoproxil fumarate . (3) including fatal cases, have been reported with the use of nucleoside analogs, including VIREAD, a component of TRUVADA. (5.1) --------------------------------CONTRAINDICATIONS----------------------------- TRUVADA is not approved for the treatment of chronic Do not use TRUVADA for pre-exposure prophylaxis in individuals with hepatitis B virus (HBV) infection. Severe acute unknown or positive HIV-1 status. TRUVADA should be used in exacerbations of hepatitis B have been reported in patients HIV-infected patients only in combination with other antiretroviral coinfected with HIV-1 and HBV who have discontinued agents. -

Targeting Fibrosis in the Duchenne Muscular Dystrophy Mice Model: an Uphill Battle
bioRxiv preprint doi: https://doi.org/10.1101/2021.01.20.427485; this version posted January 21, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Title: Targeting fibrosis in the Duchenne Muscular Dystrophy mice model: an uphill battle 2 Marine Theret1#, Marcela Low1#, Lucas Rempel1, Fang Fang Li1, Lin Wei Tung1, Osvaldo 3 Contreras3,4, Chih-Kai Chang1, Andrew Wu1, Hesham Soliman1,2, Fabio M.V. Rossi1 4 1School of Biomedical Engineering and the Biomedical Research Centre, Department of Medical 5 Genetics, 2222 Health Sciences Mall, Vancouver, BC, V6T 1Z3, Canada 6 2Department of Pharmacology and Toxicology, Faculty of Pharmaceutical Sciences, Minia 7 University, Minia, Egypt 8 3Developmental and Stem Cell Biology Division, Victor Chang Cardiac Research Institute, 9 Darlinghurst, NSW, 2010, Australia 10 4Departamento de Biología Celular y Molecular and Center for Aging and Regeneration (CARE- 11 ChileUC), Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, 8331150 12 Santiago, Chile 13 # Denotes Co-first authorship 14 15 Keywords: drug screening, fibro/adipogenic progenitors, fibrosis, repair, skeletal muscle. 16 Correspondence to: 17 Marine Theret 18 School of Biomedical Engineering and the Biomedical Research Centre 19 University of British Columbia 20 2222 Health Sciences Mall, Vancouver, British Columbia 21 Tel: +1(604) 822 0441 fax: +1(604) 822 7815 22 Email: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.01.20.427485; this version posted January 21, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. -

Viiv Healthcare Drug Class1,4: Antiretroviral Agent, Integrase
Brand Name: Tivicay Generic Name: dolutegravir Manufacturer1: ViiV Healthcare Drug Class1,4: Antiretroviral Agent, Integrase Inhibitor Labeled Uses4,5: Labeled1,4: In combination with other antiretroviral agents for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in adults and children aged 12 years and older and weighing at least 40 kg. Mechanism of Action1,2: Dolutegravir inhibits the catalytic activity of HIV integrase, which is an HIV encoded enzyme required for viral replication. Integrase is one of the three HIV-1 enzymes required for viral replication. Integration of HIV into cellular DNA is a multi-step process. First, the assembly of integrase in a stable complex with the viral DNA occurs. Second, the terminal dinucleotides from each end of the viral DNA are removed by endonucleolytic processing. Lastly, the viral DNA 3' ends are covalently linked to the cellular (target) DNA by strand transfer. The last two processes, which are catalytic, require integrase to be appropriately assembled on a specific viral DNA substrate. Inhibition of integrase by dolutegravir prevents the covalent insertion, or integration, of unintegrated linear HIV DNA into the host cell genome preventing the formation of the HIV provirus. The provirus is required to direct the production of progeny virus, so inhibiting integration prevents propagation of the viral infection. Pharmacokinetics1: Absorption: Tmax 2-3 hours Vd 17.4L T1/2 14 hours Clearance 1.0 L/h Protein Binding >98.9% Bioavailability Not established Metabolism1,2: Dolutegravir is primarily metabolized via UGT1A1 with some contribution from CYP3A. Metabolism occurs via UDP-glucuronosyltransferase (UGT)1A1 (major) and by the hepatic isoenzyme CYP3A (minor). -

Antiviral Drug Resistance
Points to Consider: Antiviral Drug Resistance Introduction Development of resistance to antimicrobial agents (including antivirals) is considered to be a natural consequence of rapid replication of microorganisms in the presence of a selective pressure. I.e. it is a natural evolutionary event and should be an expected outcome of the use of antimicrobial agents. The speed with which such resistant organisms develop, and their ability to persist in the population, will be influenced by several factors including the extent to which the antimicrobial agent is used, and the viability of the new (mutated) resistant organism. Microorganisms may also differ naturally in their sensitivity to antimicrobial agents, and the existence of such insensitivity can have an impact on emergence of resistance in two ways. (i) It provides evidence that drug resistant organisms are viable, and could therefore emerge and persist in the population in response to drug use. (ii) Use of antimicrobial agents may create an environment in which pre‐existing insensitive strains may have a selective advantage and spread. Background to drug resistant influenza viruses 1 Adamantanes (amantadine, rimantidine) The existence of viruses resistant or insensitive to this class of antiviral agent is well documented. Many of the currently circulating strains of virus (both human and animal) lack sensitivity to these agents, and clinical use of amantadine or rimantadine has been shown to select for resistant viruses in a high proportion of cases, and within 2‐3 days of starting treatmenti. Such rapid emergence of resistance during treatment may explain the reduced efficacy of rimantidine or amantadine prophylaxis when the index cases were also treatedii. -

HIV Integrase Inhibitor Pharmacogenetics and Clinical Outcomes: an Exploratory Association Study Derek E
East Tennessee State University Digital Commons @ East Tennessee State University Electronic Theses and Dissertations Student Works 8-2018 HIV Integrase Inhibitor Pharmacogenetics and Clinical Outcomes: An Exploratory Association Study Derek E. Murrell East Tennessee State University Follow this and additional works at: https://dc.etsu.edu/etd Part of the Other Pharmacy and Pharmaceutical Sciences Commons, Pharmacology Commons, and the Virus Diseases Commons Recommended Citation Murrell, Derek E., "HIV Integrase Inhibitor Pharmacogenetics and Clinical Outcomes: An Exploratory Association Study" (2018). Electronic Theses and Dissertations. Paper 3465. https://dc.etsu.edu/etd/3465 This Dissertation - Open Access is brought to you for free and open access by the Student Works at Digital Commons @ East Tennessee State University. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons @ East Tennessee State University. For more information, please contact [email protected]. HIV Integrase Inhibitor Pharmacogenetics and Clinical Outcomes: An Exploratory Association Study _____________________ A dissertation presented to the faculty of the Department of Biomedical Sciences East Tennessee State University In partial fulfillment of the requirements for the degree Doctor of Philosophy in Biomedical Sciences, Pharmaceutical Sciences Concentration _____________________ by Derek Edward Murrell August 2018 _____________________ Sam Harirforoosh, PharmD, PhD, Chair Jonathan Moorman, MD, PhD David Roane, PhD Robert Schoborg, PhD Zhi Qiang Yao, MD, PhD Keywords: Integrase Strand Transfer Inhibitor, Dolutegravir, Elvitegravir, Raltegravir, Pharmacogenetics, HIV, Renal, Hepatic, Adverse events ABSTRACT HIV Integrase Inhibitor Pharmacogenetics and Clinical Outcomes: An Exploratory Association Study by Derek Edward Murrell As HIV is now primarily a chronic condition, treatment is given life-long with changes as necessitated by alterations in tolerability and efficacy.