REYATAZ Rx Only
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Treatment-Drugs
© National HIV Curriculum PDF created September 23, 2021, 9:14 am Stavudine (Zerit) Table of Contents Stavudine Zerit Summary Drug Summary Key Clinical Trials Resistance Key Drug Interactions Drug Summary Stavudine, an early nucleoside reverse transcriptase inhibitor (NRTI), was used as part of combination antiretroviral therapy for years, but now has become obsolete and replaced by better-tolerated and safer options. Stavudine poses risk of serious toxicity, including peripheral neuropathy (which can be permanent), pancreatitis, lipoatrophy, and lactic acidosis. Fatal and nonfatal cases of pancreatitis and lactic acidosis have been reported, especially when stavudine was combined with didanosine. According to the Adult and Adolescent ARV Guidelines, stavudine is no longer recommended for the treatment of HIV infection due to potential severe toxicity. Further, all persons currently taking stavudine should be strongly encouraged to switch to a safer medication. The sale and distribution of all strengths of stavudine will be discontinued and removed from the market in the United States in 2020. Key Clinical Trials Stavudine was studied for treatment-naïve patients as part of triple therapy, such as with lamivudine plus indinavir [START I], lamivudine plus lopinavir-ritonavir [M98-863], and lamivudine plus efavirenz [DART II]. Several studies demonstrated benefits of switching stavudine to newer NRTI agents, such as tenofovir disoproxil fumarate; the switch led to decreased rates of metabolic complications and mitochondrial toxicity [903E, SNAP, and ACTG 5142]. Resistance For a listing of the most common clinically significant mutations associated with stavudine (d4T) resistance, see the NRTI Resistance Notes on the Stanford University HIV Drug Resistance Database. Page 1/2 Key Drug Interactions For complete information on stavudine-related drug interactions, see the Drug Interactions section in the Stavudine (Zerit) Prescribing Information. -

Eparate Formulations According to the Prescribed Dosing Recommendations for These Products
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Lamivudine/Zidovudine Teva 150 mg/300 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 150 mg lamivudine and 300 mg zidovudine. For the full list of excipients see section 6.1. 3. PHARMACEUTICAL FORM Film-coated tablet White, capsule shaped, biconvex, film-coated scored tablet – engraved with “L/Z” on one side and “150/300” on the other side. The tablet can be divided into equal halves. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Lamivudine/Zidovudine Teva is indicated in antiretroviral combination therapy for the treatment of Human Immunodeficiency Virus (HIV) infection (see section 4.2). 4.2 Posology and method of administration Therapy should be initiated by a physician experienced in the management of HIV infection. Lamivudine/Zidovudine Teva may be administered with or without food. To ensure administration of the entire dose, the tablet(s) should ideally be swallowed without crushing. For patients who are unable to swallow tablets, tablets may be crushed and added to a small amount of semi-solid food or liquid, all of which should be consumed immediately (see section 5.2). Adults and adolescents weighing at least 30 kg: the recommended oral dose of Lamivudine/Zidovudine Teva is one tablet twice daily. Children weighing between 21 kg and 30 kg: the recommended oral dose of Lamivudine/Zidovudine Teva is one-half tablet taken in the morning and one whole tablet taken in the evening. Children weighing from 14 kg to 21 kg: the recommended oral dose of Lamivudine/Zidovudine Teva is one-half tablet taken twice daily. -

Revised 4/1/2021 GEORGIA MEDICAID FEE-FOR-SERVICE HIV
GEORGIA MEDICAID FEE-FOR-SERVICE HIV-AIDS PA SUMMARY Preferred (may not be all inclusive) Non-Preferred Abacavir generic Abacavir/lamivudine/zidovudine generic Abacavir/lamivudine generic Aptivus (tipranavir) Complera (emtricitabine/rilpivirine/tenofovir disoproxil Atazanavir capsules generic fumarate) Atripla (efavirenz/emtricitabine/tenofovir disoproxil Crixivan (indinavir) fumarate) Biktarvy (bictegravir/emtricitabine/tenofovir Delstrigo (doravirine/lamivudine/tenofovir disoproxil alafenamide) fumarate) Cimduo (lamivudine/tenofovir disoproxil fumarate) Fuzeon (enfuvirtide) Descovy (emtricitabine/tenofovir alafenamide) Intelence (etravirine) Dovato Invirase (saquinavir) Edurant (rilpivirine)* Lexiva (fosamprenavir) Efavirenz tablets generic Nevirapine extended-release generic Emtriva (emtricitabine) Norvir Powder (ritonavir) Epivir solution (lamivudine) Pifeltro (doravirine) Evotaz (atazanavir/cobicistat)* Reyataz Powder (atazanavir) Genvoya (elvitegravir/cobicistat/emtricitabine/ Ritonavir tablets generic tenofovir alafenamide) Isentress and Isentress HD (raltegravir)* Rukobia (fostemsavir) Juluca (dolutegravir/rilpivirine) Selzentry (maraviroc) Kaletra (lopinavir/ritonavir) Stavudine generic^ Stribild (elvitegravir/cobicistat/emtricitabine/ tenofovir Lamivudine generic disoproxil fumarate) Symfi (efavirenz 600 mg/lamivudine/tenofovir Lamivudine/zidovudine generic disoproxil fumarate) Symfi Lo (efavirenz 400 mg/lamivudine/tenofovir Nevirapine immediate-release tablets generic disoproxil fumarate) Norvir (ritonavir) Temixys (lamivudine/tenofovir -
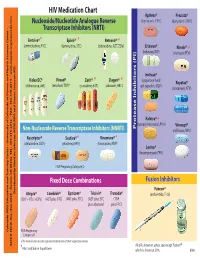
HIV Medication Chart Aptivus® Prezista® Nucleoside/Nucleotide Analogue Reverse (Tipranavir, TPV) (Darunavir, DRV) Transcriptase Inhibitors (NRTI)
HIV Medication Chart Aptivus® Prezista® Nucleoside/Nucleotide Analogue Reverse (tipranavir, TPV) (darunavir, DRV) Transcriptase Inhibitors (NRTI) Emtriva®* Epivir® * Retrovir® * (emtricitabine, FTC) (lamivudine, 3TC) (zidovudine, AZT, ZDV) Crixivan® Norvir® * (indinavir, IDV) (ritonavir, RTV) Invirase® Videx EC® Viread® Zerit® Ziagen® * * (saquinavir hard Reyataz® (didanosine, ddl) (tenofovir,TDF)* (stavudine, d4T) (abacavir, ABC) gel capsules, SQV) (atazanavir, ATV) Kaletra® * (lopinavir/ritonavir, LPV/r) Viracept® Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTI) Inhibitors (PI) Protease (nelfinavir, NFV) Rescriptor® Sustiva®* Viramune® * (delavirdine, DLV) (efavirenz, EFV) (nevirapine, NVP) Lexiva® (fosamprenavir, FPV) FDA Pregnancy Category D Fixed Dose Combinations Fusion Inhibitors Fuzeon® Developed by MeriLou Johnson, MeriLou by Developed Johnson, MSW, and Steven MPA MD Atripla® Combivir® Epzicom® Trizivir® Truvada® (enfuvirtide,T-20) (TDF+FTC+EFV) (AZT plus 3TC) (ABC plus 3TC) (AZT plus 3TC (TDF plus abacavir) plus FTC) Colorado AIDS Education and Training Center • University of Colorado at Denver and Health Sciences Center • Center and Health Sciences Denver at of Colorado • University Center Training and AIDS Education Colorado FDA Pregnancy Category D ®The brands listed are the registered trademarks of their respective owners. 4200 E. Ave., Ninth TEL: A089 • Denver, Box 80262 CO 303-315-2512 • FAX: • 303-315-2514 • www.mpaetc.org/colorado.htm All pills shown in actual size except Fuzeon® *Also available in liquid form. which is shown at 50%. 8/06 Medication Schedule Name______________________________ Date___________ Number of Time of day you ar to take TOTAL Name of Medication pills to take this medicine Food Interactions Side Effects number of each time pills each day With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ Discontinued Medications or Formulations Helpful Hints: • Refill prescriptions before you run out. -

Truvada (Emtricitabine / Tenofovir Disoproxil)
Pre-exposure Prophylaxis (2.3) HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Recommended dose in HIV-1 uninfected adults: One tablet TRUVADA safely and effectively. See full prescribing information (containing 200 mg/300 mg of emtricitabine and tenofovir for TRUVADA. disoproxil fumarate) once daily taken orally with or without food. (2.3) TRUVADA® (emtricitabine/tenofovir disoproxil fumarate) tablets, for oral use Recommended dose in renally impaired HIV-uninfected Initial U.S. Approval: 2004 individuals: Do not use TRUVADA in HIV-uninfected individuals if CrCl is below 60 mL/min. If a decrease in CrCl is observed in WARNING: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH uninfected individuals while using TRUVADA for PrEP, evaluate STEATOSIS, POST-TREATMENT ACUTE EXACERBATION OF potential causes and re-assess potential risks and benefits of HEPATITIS B, and RISK OF DRUG RESISTANCE WITH USE OF continued use. (2.4) TRUVADA FOR PrEP IN UNDIAGNOSED HIV-1 INFECTION -----------------------DOSAGE FORMS AND STRENGTHS------------------- See full prescribing information for complete boxed warning. Tablets: 200 mg/300 mg, 167 mg/250 mg, 133 mg/200 mg, and 100 Lactic acidosis and severe hepatomegaly with steatosis, mg/150 mg of emtricitabine and tenofovir disoproxil fumarate . (3) including fatal cases, have been reported with the use of nucleoside analogs, including VIREAD, a component of TRUVADA. (5.1) --------------------------------CONTRAINDICATIONS----------------------------- TRUVADA is not approved for the treatment of chronic Do not use TRUVADA for pre-exposure prophylaxis in individuals with hepatitis B virus (HBV) infection. Severe acute unknown or positive HIV-1 status. TRUVADA should be used in exacerbations of hepatitis B have been reported in patients HIV-infected patients only in combination with other antiretroviral coinfected with HIV-1 and HBV who have discontinued agents. -

Download Article PDF/Slides
New Antiretrovirals in Development: Reprinted from The PRN Notebook,™ june 2002. Dr. James F. Braun, Editor-in-Chief. Tim Horn, Executive Editor. Published in New York City by the Physicians’ Research Network, Inc.,® John Graham Brown, Executive Director. For further information and other articles The View in 2002 available online, visit http://www.PRN.org All rights reserved. © june 2002. Roy “Trip” Gulick, md, mph Associate Professor of Medicine, Weill Medical College of Cornell University Director, Cornell Clinical Trials Unit, New York, New York Summary by Tim Horn Edited by Scott Hammer, md espite the fact that 16 antiretro- tiviral activity of emtricitabine was estab- Preliminary results from two random- virals are approved for use in the lished, with total daily doses of 200 mg or ized studies—FTC-302 and FTC-303—were United States, there is an indis- more producing the greatest median viral reported by Dr. Charles van der Horst and putable need for new anti-hiv com- load suppression: 1.72-1.92 log. Based on his colleagues at the 8th croi, held in Feb- pounds that have potent and these data, a once-daily dose of 200 mg ruary 2001 in Chicago (van der Horst, durable efficacy profiles, unique re- was selected for further long-term clinical 2001). FTC-302 was a blinded comparison sistance patterns, patient-friendly dosing study. “This is what we’re looking forward of emtricitabine and lamivudine, both in schedules, and minimal toxicities. To pro- to with emtricitabine,” commented Dr. combination with stavudine (Zerit) and vide prn with a glimpse of drugs current- Gulick. -

Product Monograph for CELSENTRI
PRODUCT MONOGRAPH PrCELSENTRI maraviroc Tablets 150 and 300 mg CCR5 antagonist ViiV Healthcare ULC 245, boulevard Armand-Frappier Laval, Quebec H7V 4A7 Date of Revision: July 05, 2019 Submission Control No: 226222 © 2019 ViiV Healthcare group of companies or its licensor. Trademarks are owned by or licensed to the ViiV Healthcare group of companies. Page 1 of 60 Table of Contents PART I: HEALTH PROFESSIONAL INFORMATION.........................................................3 SUMMARY PRODUCT INFORMATION ........................................................................3 INDICATIONS AND CLINICAL USE..............................................................................3 CONTRAINDICATIONS ...................................................................................................3 WARNINGS AND PRECAUTIONS..................................................................................4 ADVERSE REACTIONS....................................................................................................9 DRUG INTERACTIONS ..................................................................................................19 DOSAGE AND ADMINISTRATION..............................................................................28 OVERDOSAGE ................................................................................................................31 ACTION AND CLINICAL PHARMACOLOGY ............................................................31 STORAGE AND STABILITY..........................................................................................36 -

DESCOVY, and Upon Diagnosis of These Highlights Do Not Include All the Information Needed to Use Any Other Sexually Transmitted Infections (Stis)
HIGHLIGHTS OF PRESCRIBING INFORMATION once every 3 months while taking DESCOVY, and upon diagnosis of These highlights do not include all the information needed to use any other sexually transmitted infections (STIs). (2.2) DESCOVY safely and effectively. See full prescribing information • Recommended dosage: for DESCOVY. • Treatment of HIV-1 Infection: One tablet taken once daily with or ® without food in patients with body weight at least 25 kg. (2.3) DESCOVY (emtricitabine and tenofovir alafenamide) tablets, for • HIV-1 PrEP: One tablet taken once daily with or without food in oral use individuals with body weight at least 35 kg. (2.4) Initial U.S. Approval: 2015 • Renal impairment: DESCOVY is not recommended in individuals with WARNING: POST-TREATMENT ACUTE EXACERBATION OF estimated creatinine clearance below 30 mL per minute. (2.5) HEPATITIS B and RISK OF DRUG RESISTANCE WITH USE ----------------------DOSAGE FORMS AND STRENGTHS-------------------- OF DESCOVY FOR HIV-1 PRE-EXPOSURE PROPHYLAXIS Tablets: 200 mg of FTC and 25 mg of TAF (3) (PrEP) IN UNDIAGNOSED EARLY HIV-1 INFECTION See full prescribing information for complete boxed warning. -------------------------------CONTRAINDICATIONS------------------------------ DESCOVY for HIV-1 PrEP is contraindicated in individuals with Severe acute exacerbations of hepatitis B (HBV) have been unknown or positive HIV-1 status. (4) reported in HBV-infected individuals who have discontinued products containing emtricitabine (FTC) and/or tenofovir -----------------------WARNINGS AND PRECAUTIONS----------------------- disoproxil fumarate (TDF), and may occur with • Comprehensive management to reduce the risk of sexually discontinuation of DESCOVY. Hepatic function should be transmitted infections (STIs), including HIV-1, when DESCOVY is monitored closely in these individuals. -

(12) United States Patent (10) Patent No.: US 7,732,399 B2 Goldenberg Et Al
US007732399B2 (12) United States Patent (10) Patent No.: US 7,732,399 B2 Goldenberg et al. (45) Date of Patent: *Jun. 8, 2010 (54) SUSTAINED RELEASE FORMULATIONS 2002fO151582 A1 10, 2002 Dou et al. 2003/O190307 A1* 10, 2003 DiBiase et al. ............. 424,856 (75) Inventors: Merrill S. Goldenberg, Thousand Oaks, 2004/0142048 A1 7/2004 Moore et al. CA (US); Jian Hua Gu, Thousand Oaks 2005/0180925 A1* 8/2005 Chaudry ...................... 424/46 CA (US s s 2005/0215470 A1 9/2005 Ng et al. FOREIGN PATENT DOCUMENTS (73) Assignee: Amgen Inc., Thousand Oaks, CA (US) GB 92.9405 A 6, 1963 (*) Notice: Subject to any disclaimer, the term of this GB 1234.805. A 6, 1971 patent is extended or adjusted under 35 W W 3. t E. U.S.C. 154(b) by 193 days. WO WO99,04764 2, 1999 This patent is Subject to a terminal dis- W W 995, 239, claimer. WO WO 2004/O12522 2, 2004 (21) Appl. No.: 11/847,984 OTHER PUBLICATIONS 1-1. Yan et al. Identification of histatins as tannin-binding proteins in (22) Filed: Aug. 30, 2007 human saliva. Biochemical Journal. 1995, vol. 311, pp. 341-347.* O O Charlton, A. J. et al., “Polyphenol/Peptide Binding and Precipita (65) Prior Publication Data tion.” J. Agric. Food Chem. 50, pp. 1593-1601 (2002); published by US 2007/0292506 A1 Dec. 20, 2007 Chasin,American M.. Chemical “Biodegradable Society. Polymers for Controlled Drug Deliv O O ery,” J.O. Hollinger Editor, Biomedical Applications of Synthetic Related U.S. Application Data Biodegradable Polymers CRC, Boca Raton, FL (1995), pp. -

Lamivudine in Combination with Zidovudine, Stavudine, Or Didanosine in Patients with HIV-1 Infection
Lamivudine in combination with zidovudine, stavudine, or didanosine in patients with HIV-1 infection. A randomized, double-blind, placebo-controlled trial Daniel R. Kuritzkes*, Ian Marschner†, Victoria A. Johnson‡, Roland Bassett†, Joseph J. Eron§, Margaret A. FischlII, Robert L. Murphy¶, Kenneth Fife**, Janine Maenza††, Mary E. Rosandich*, Dawn Bell‡‡, Ken Wood§§, Jean-Pierre Sommadossi‡, Carla PettinelliII II and the National Institute of Allergy and Infectious Disease AIDS Clinical Trials Group Protocol 306 Investigators Objective: To study the antiviral activity of lamivudine (3TC) plus zidovudine (ZDV), didanosine (ddI), or stavudine (d4T). Design: Randomized, placebo-controlled, partially double-blinded multicenter study. Setting: Adult AIDS Clinical Trials Units. Patients: Treatment-naive HIV-infected adults with 200–600 × 106 CD4 T lymphocytes/l. Interventions: Patients were openly randomized to a d4T or a ddI limb, then randomized in a blinded manner to receive: d4T (80 mg/day), d4T plus 3TC (300 mg/day), or ZDV (600 mg/day) plus 3TC, with matching placebos; or ddI (400 mg/day), ddI plus 3TC (300 mg/day), or ZDV (600 mg/day) plus 3TC, with matching placebos. After 24 weeks 3TC was added for patients assigned to the monotherapy arms. Main outcome measure: The reduction in plasma HIV-1 RNA level at weeks 24 and 48. From the *University of Colorado Health Sciences Center and Veterans Affairs Medical Center, Denver, Colorado, the †Center for Biostatistics in AIDS Research, Harvard School of Public Health, Boston, Massachusetts, the -

Minutes of PRAC Meeting on 09-12 July 2018
6 September 2018 EMA/PRAC/576790/2018 Inspections, Human Medicines Pharmacovigilance and Committees Division Pharmacovigilance Risk Assessment Committee (PRAC) Minutes of the meeting on 09-12 July 2018 Chair: June Raine – Vice-Chair: Almath Spooner Health and safety information In accordance with the Agency’s health and safety policy, delegates were briefed on health, safety and emergency information and procedures prior to the start of the meeting. Disclaimers Some of the information contained in the minutes is considered commercially confidential or sensitive and therefore not disclosed. With regard to intended therapeutic indications or procedure scope listed against products, it must be noted that these may not reflect the full wording proposed by applicants and may also change during the course of the review. Additional details on some of these procedures will be published in the PRAC meeting highlights once the procedures are finalised. Of note, the minutes are a working document primarily designed for PRAC members and the work the Committee undertakes. Note on access to documents Some documents mentioned in the minutes cannot be released at present following a request for access to documents within the framework of Regulation (EC) No 1049/2001 as they are subject to on- going procedures for which a final decision has not yet been adopted. They will become public when adopted or considered public according to the principles stated in the Agency policy on access to documents (EMA/127362/2006, Rev. 1). 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2018. -

EVOTAZ (Atazanavir Or Cobicistat) to Pregnant Rats and Rabbits (See Data)
HIGHLIGHTS OF PRESCRIBING INFORMATION • Assess creatinine clearance (CLcr) before initiating treatment. Consider These highlights do not include all the information needed to use EVOTAZ alternative medications that do not require dosage adjustments in patients safely and effectively. See full prescribing information for EVOTAZ. with renal impairment. (5.3) • When cobicistat, a component of EVOTAZ, is used in combination with a EVOTAZ (atazanavir and cobicistat) tablet, for oral use tenofovir disoproxil fumarate (tenofovir DF)-containing regimen, cases of Initial U.S. Approval: 2015 acute renal failure and Fanconi syndrome have been reported. (5.4) • When used with tenofovir DF, assess urine glucose and urine protein at ---------------------------RECENT MAJOR CHANGES-------------------------- baseline and monitor CLcr, urine glucose, and urine protein. Monitor serum Indications and Usage phosphorus in patients with or at risk for renal impairment. Coadministration Indications (1.1) 07/2020 with tenofovir DF is not recommended in patients with CLcr below 70 Dosage and Administration mL/min or in patients also receiving a nephrotoxic agent. (5.4) Laboratory Testing Prior to Initiation and During • Chronic kidney disease has been reported during postmarketing surveillance Treatment with EVOTAZ (2.1) 04/2020 in patients with HIV-1 infection treated with atazanavir, with or without Recommended Dosage (2.2) 07/2020 ritonavir. Consider alternatives in patients at high risk for renal disease or Not Recommended During Pregnancy (2.5) 04/2020 with preexisting renal disease. Monitor renal laboratory tests prior to therapy Contraindications (4) 04/2020 and during treatment with EVOTAZ. Consider discontinuation of EVOTAZ Warnings and Precautions in patients with progressive renal disease. (5.5) Immune Reconstitution Syndrome (5.11) 07/2020 • Nephrolithiasis and cholelithiasis have been reported.