Finned Pilot Whales Reduce Sound Exposure from Naval Sonar?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biomechanics of Safe Ascents Workshop
PROCEEDINGS OF BIOMECHANICS OF SAFE ASCENTS WORKSHOP — 10 ft E 30 ft TIME AMERICAN ACADEMY OF UNDERWATER SCIENCES September 25 - 27, 1989 Woods Hole, Massachusetts Proceedings of the AAUS Biomechanics of Safe Ascents Workshop Michael A. Lang and Glen H. Egstrom, (Editors) Copyright © 1990 by AMERICAN ACADEMY OF UNDERWATER SCIENCES 947 Newhall Street Costa Mesa, CA 92627 All Rights Reserved No part of this book may be reproduced in any form by photostat, microfilm, or any other means, without written permission from the publishers Copies of these Proceedings can be purchased from AAUS at the above address This workshop was sponsored in part by the National Oceanic and Atmospheric Administration (NOAA), Department of Commerce, under grant number 40AANR902932, through the Office of Undersea Research, and in part by the Diving Equipment Manufacturers Association (DEMA), and in part by the American Academy of Underwater Sciences (AAUS). The U.S. Government is authorized to produce and distribute reprints for governmental purposes notwithstanding the copyright notation that appears above. Opinions presented at the Workshop and in the Proceedings are those of the contributors, and do not necessarily reflect those of the American Academy of Underwater Sciences PROCEEDINGS OF THE AMERICAN ACADEMY OF UNDERWATER SCIENCES BIOMECHANICS OF SAFE ASCENTS WORKSHOP WHOI/MBL Woods Hole, Massachusetts September 25 - 27, 1989 MICHAEL A. LANG GLEN H. EGSTROM Editors American Academy of Underwater Sciences 947 Newhall Street, Costa Mesa, California 92627 U.S.A. An American Academy of Underwater Sciences Diving Safety Publication AAUSDSP-BSA-01-90 CONTENTS Preface i About AAUS ii Executive Summary iii Acknowledgments v Session 1: Introductory Session Welcoming address - Michael A. -

Stiddmil.Com POWER POD RNAV2 SIMULATOR
DPD2 • RNAV2 • AP2 • OM2 • AC2 • POWER POD • CP2 CATALOG 22 POWER POD NEW! RNAV2 SIMULATOR Manned & Autonomous Vehicles with Navigation, Control & Communications for EOD and Maritime SOF stiddmil.com MADE IN U.S.A. Manned or Autonomous... The “All-In-One” Vehicle Moving easily between manned and autonomous roles, STIDD’s new generation of propulsion vehicles provide operators innovative options for an increasingly complex underwater environment. Over the past 20 years, STIDD built its Submersible line and flagship product, the Diver Propulsion Device (DPD), around the basic idea that divers would prefer riding a vehicle instead of swimming. Today, STIDD focuses on another simple, but transformative goal: design, develop, and integrate the most advanced Precision Navigation, Control, Communications, and Automation Technology available into the DPD to make that ride easier, more effective, and when desired . RIDERLESS! DPD2 - Manned Mode 1 DPD2 - OM2 Mode Precision Navigation, Control, Communications & Automation System for the DPD POWERED BY RNAV2 GREENSEA Building on the legacy of its Diver Propulsion Device (DPD), the most widely used combat vehicle of its kind, STIDD designed and developed a system of DPD Navigation, Control, Communications, and Automation features which enable a seamless transition between Manned and fully Autonomous modes. RNAV2 was developed by STIDD partnering with Greensea as the backbone of this capability. RNAV2 is powered by Greensea’s patent-pending OPENSEA™ operating platform, which not only enables RNAV2’s open architecture, but also seamlessly integrates STIDD’s OM2/AP2 Diver Assist /S2 Sonar/ AC2 Communications products into an intuitive, easy to use, autonomous system. When fully configured with the Precision Navigation, Control & Automation System including RNAV2/ OM2/AP2/S2/AC2, any DPD easily transitions between Manned, DPD with RNAV2 Installed Semi-Autonomous, and Full-Autonomous modes. -

Atmos Elite Owner's Guide, Doc
OR ATMOS ELITE DIVE COMPUTER OWNER'S GUIDE LIMITED TWO-YEAR WARRANTY For details, refer to the Product Warranty Registration Card provided. COPYRIGHT NOTICE This owners guide is copyrighted, all rights are reserved. It may not, in whole or in part, be copied, photocopied, reproduced, translated, or reduced to any electronic medium or machine readable form without prior consent in writ- ing from AERIS / 2002 Design. Atmos Elite Owner's Guide, Doc. No. 12-7156 © 2002 Design 2003 San Leandro, Ca. USA 94577 TRADEMARK NOTICE AERIS, the AERIS logo, Atmos Elite, and the Atmos Elite logo are all registered and unregistered trademarks of AERIS. All rights are reserved. PATENT NOTICE U.S. Patents have been issued, or applied for, to protect the following design features: Dive Time Remaining (U.S. Patent no. 4,586,136), Data Sensing and Processing Device (U.S. Patent no. 4,882,678), and Ascent Rate Indicator (U.S. Patent no. 5,156,055). User Setable Display (U.S. Patent no. 5,845,235) is owned by Suunto Oy (Finland). DECOMPRESSION MODEL The programs within the Atmos Elite simulate the absorption of nitrogen into the body by using a mathematical model. This model is merely a way to apply a limited set of data to a large range of experiences. The Atmos Elite dive computer model is based upon the latest research and experiments in decompression theory. Still, using the Atmos Elite, just as using the U.S. Navy (or other) No Decompression Tables, is no guarantee of avoiding decompression sickness, i.e. the bends. Every divers physiology is different, and can even vary from day to day. -

The Effects of Warm and Cold Water Scuba Finning on Cardiorespiratory Responses and Energy Expenditure
AN ABSTRACT OF THE THESISOF in Caron Lee Louise Shake for the degreeof Doctor of Philosophy Education presented on April 5, 1989. Scuba Finning on Title: The Effects of Warm and Cold Water Cardiorespiratory Responses and EnergyExpenditure Redacted for privacy Abstract approved: cardiorespiratory and energy This study was designed to determine finning at expenditure responses elicited byrecreational divers while and warm (29°C) water a submaximal intensity(35% max) in cold (18°C) to par- with and without wet suits. Male divers (15) volunteered exercise ticipate in five experimentalprocedures. A maximal graded in 29°C tethered finning test, two submaximal(30 min.) finning tests tests with and without wet suits, and twosubmaximal (30 min.) finning The variables in 18°C with and without wetsuits were performed. (VE), measured were: breathing frequency(BF), minute ventilation (RER), heart rate oxygen consumption (V02)respiratory exchange ratio (HR), and core temperature (CT). Caloric expenditure (kcal) was calculated from RER and V02. A Four-Way ANOVA andrepeated measures 0.05) Two-Way design was used to analyze the data. A significant (p < A significant (p < (suit x time) interaction wasrevealed for BF. 0.01) Three-Way (suit x temp. x time)interaction was revealed forVE, V02, RER, HR, and CT. An inverse relationship exists betweenBF and VE when comparing dives with and without suits. Diving in 18°C with suitselicited higher BF and lower VE than diving in 29°Cwithout suits. V02 increased significantly during threeof the four dives. Diving without suits elicited higher V02values though this was not significant in every case. Diving in a cold environmentelicited lower RER re- higher V02 and VE. -

FIU-DOM-01 Revision-1 12/2019 10
FIU-DOM-01 Revision -1 12/2019 1 11200 SW 8th Street, Miami Florida, 33199 http://www.fiu.edu TABLE of CONTENTS Section 1.00 GENERAL POLICY 6 1.10 Diving Standards 6 1.20 Operational Control 7 1.30 Consequence of Violation of Regulations by divers 9 1.40 Job Safety Analysis 9 1.50 Dive Team Briefing 10 1.60 Record Maintenance 10 Section 2.00 MEDICAL STANDARDS 11 2.10 Medical Requirements 11 2.20 Frequency of Medical Evaluations 11 2.30 Information Provided Examining Physician 11 2.40 Content of Medical Evaluations 11 2.50 Conditions Which May Disqualify Candidates from Diving (Adapted from Bove, 1998) 11 2.60 Laboratory Requirements for Diving Medical Evaluation and Intervals 12 2.70 Physician's Written Report 13 Section 3.00 ENTRY-LEVEL REQUIRMENTS 14 3.10 General Policy 14 Section 4.00 DIVER QUALIFICATION 14 4.10 Prerequisites 14 4.20 Training 15 4.30 FIU Working Diver Qualification 18 4.40 External (Non-FIU Employee) Diver Qualifications 18 4.50 Depth Certifications 22 4.60 Continuation of FIU Working Diver Certification 22 4.70 Revocation of Certification or Designation 23 4.80 Requalification After Revocation of Diving Privileges 23 4.90 Guest Diver 23 Section 5.00 DIVING REGULATIONS FOR SCUBA (OPEN CIRCUIT, COMPRESSED AIR) 24 5.10 Introduction 24 5.20 Pre-Dive Procedures 24 5.30 Diving Procedures 25 5.40 Post-Dive Procedures 30 5.50 Emergency Procedures 30 5.60 Flying After Diving or Ascending to Altitude (Over 1000 feet) 30 5.70 Record Keeping Requirements 30 FIU-DOM-01 Revision-1 12/2019 2 Section 6.00 SCUBA DIVING EQUIPMENT 32 -

Mares Buyer's Guide
buyer’s guide MISSION / INTRO COMPANY / PROFILE HOW TO READ In 1949, Ludovico Mares designed and manufactured his first masks and Would Ludovico Mares ever have imagined that over the course of 68 years The 2017 Mares catalog contains all of Mares’ products: a complete collection of the latest equipment, filled with innovative product features, spearguns with one purpose in mind: to share his extreme passion for his small factory in Rapallo would become the worldwide leader in the pro- designed to meet and satisfy the needs and dreams of every individual diver. the sea and diving with the rest of the world. At the beginning, Mares was duction and distribution of diving equipment? This catalogue has been developed specifically to help you the dealer make the best choices when selecting which products and technologies will just a small factory in Rapallo; today, more than 68 years later, the Italian Mares was founded in 1949 by former Istrian diver Ludovico Mares, who best fulfill your customer’s needs. based company dominates the scuba diving world with leading design served in the Austrian Navy during World War I. and technology. Over the past six decades, Mares has come a long way by Mares quickly became a small industrial company with a continuous in- Keep on reading and enjoy the new 2017 Mares Collection. achieving new goals and taking diving to new extreme heights and depths. crease in sales and never an absence of ideas for new and improved Mares represents only the best in dive products. products. As the passion for diving grew around the world in the late ‘60s, Over the past 68 years, Mares has become the worldwide leader in the company expanded into the European diving and snorkeling market. -

Diving Safe Practices Manual
Diving Safe Practices Manual Underwater Inspection Program U.S. Department of the Interior February 2021 Mission Statements The Department of the Interior conserves and manages the Nation’s natural resources and cultural heritage for the benefit and enjoyment of the American people, provides scientific and other information about natural resources and natural hazards to address societal challenges and create opportunities for the American people, and honors the Nation’s trust responsibilities or special commitments to American Indians, Alaska Natives, and affiliated island communities to help them prosper. The mission of the Bureau of Reclamation is to manage, develop, and protect water and related resources in an environmentally and economically sound manner in the interest of the American public. Diving Safe Practices Manual Underwater Inspection Program Prepared by R. L. Harris (September 2006) Regional Dive Team Leader and Chair Reclamation Diving Safety Advisory Board Revised by Reclamation Diving Safety Advisory Board (February 2021) Diving Safe Practices Manual Contents Page Contents .................................................................................................................................. iii 1 Introduction .............................................................................................................. 1 1.1 Use of this Manual ............................................................................................. 1 1.2 Diving Safety ..................................................................................................... -

Ufdssp - Project Dive Log
UFDSSP - PROJECT DIVE LOG UFDSSP standards and AAUS standards require that the individual diver submit a dive log at least every two months to document their diving activity. The UF permits (encourages) use of a single dive log prepared by the dive master on each dive filed by dive project. The standard log form has been simplified in an effort to facilitate accurate completion. However, a few logs have been submitted with gross inaccuracies. To further make the log a document for accountability, the latest revision has a place for both the dive master and the diver to sign indicating that the log reflects accurately their dive activity. If a computer is used, Please submit Log with pertinent data The following items are minimally needed on all dive logs submitted: Dive project number (assigned when project approved) 1. Date 2. Dive Location (location/boat/as on dive plan, i.e. Swannee Regional Reef, #24, Menippe,) 3. Dive Master/Lead Diver* Person providing oversight and safety accountability 4. Site # If multiple sites used, indicate number or description of site 5. Equipment used/gas used (SCUBA, SAS/nitrox40<air if assumed if left blank>, and wet/dry suit, Bandmask) Computer - type/brand 6. Environmental conditions(reflect sea state, visibility,bottom type, & current) 7. Tables used (USN, USNEAD, DCIEM, RPLN, etc) 8. Dive Plan - tentative plan for dive represent time & depth & Surface Intervals 10. Dive Team - expected 2 divers, may be more, or if dive project authorized - single diver with tender/standby diver (specify) 11. PSI - Cylinder gas used (80/1500) - SAS=1... -

Resting Oxygen Consumption Rates in Divers Using Diver Propulsion Devices Adam J
University of South Florida Scholar Commons Graduate Theses and Dissertations Graduate School 10-29-2008 Resting Oxygen Consumption Rates in Divers Using Diver Propulsion Devices Adam J. Smith University of South Florida Follow this and additional works at: https://scholarcommons.usf.edu/etd Part of the American Studies Commons Scholar Commons Citation Smith, Adam J., "Resting Oxygen Consumption Rates in Divers Using Diver Propulsion Devices" (2008). Graduate Theses and Dissertations. https://scholarcommons.usf.edu/etd/502 This Thesis is brought to you for free and open access by the Graduate School at Scholar Commons. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. RestingOxygenConsumptionRatesinDiversUsingDiverPropulsionDevices by AdamJ.Smith Athesissubmittedinpartialfulfillment oftherequirementsforthedegreeof MasterofScienceinBiomedicalEngineering DepartmentofChemical&BiomedicalEngineering CollegeofEngineering UniversityofSouthFlorida MajorProfessor:WilliamE.LeeIII,Ph.D. JohnR.Clarke,Ph.D. RolandD.Shytle,Ph.D. DateofApproval: October29,2008 Keywords:OxygenConsumption,Diving,Rebreather,PropagationofError, InjectionRate,Nitrox,Semiclosed ©Copyright2008,AdamJ.Smith Dedication Thisthesisisdedicatedtomyfamilywhohavelovedandsupportedme throughoutmystudies.Iamblessedtohavesuchgreatrolemodelsasmy parents. Acknowledgments First,IwouldliketoexpressthedeepestofgratitudetoDr.JohnClarke. -

Use of Surface-Supplied Gas for Scientific Diving
In: Pollock NW, ed. Diving for Science 2011. Proceedings of the American Academy of Underwater Sciences 30th Symposium. Dauphin Island, AL: AAUS; 2011. Use of Surface-Supplied Gas for Scientific Diving Alan Humphrey1*, Scott Grossman2, Jonathan McBurney2, Sean Sheldrake3 1 USEPA, Environmental Response Team, 2890 Woodbridge Avenue, Edison, NJ 08837, USA [email protected] 2 Lockheed Martin SERAS, 2890 Woodbridge Avenue, Edison, NJ 08837, USA 3 USEPA, Region 10, 1200 6th Avenue, Suite 900, Mailstop ECL-110, Seattle, WA 98101, USA *corresponding author Abstract Many scientific diving entities face logistical challenges in their data collection, including high currents, low visibility, finite air supply, and high entanglement areas. This paper and presentation will discuss standard practices for the use of surface-supplied breathing gas for scientific applications to enhance both safety and efficiency of the scientific diver. The paper will examine the relevant regulatory requirements and applicable standards of the federal dive community (e.g., NOAA, EPA) relative to the application of scientific diving, as well as some pros/cons for the scientific diver of tether vs. surface-supply vs. free swimming. Lastly, the paper will make recommendations to advance the surface-supplied breathing gas mode within the scientific diving community. Keywords: contaminated water protocols, epa diving, federal dive programs polluted diving, surface-supplied diving, surface-supplied gas Introduction Surface-supplied diving is unique compared to free swimming scientific divers' more routine use of self-contained underwater breathing apparatus (SCUBA) to accomplish their objectives. Using surface-supplied air, the diver is provided a virtually unlimited supply of breathing gas from tanks or a compressor on the surface through a three-part umbilical consisting of a breathing gas hose, a pneumofathometer (pneumo) hose and communication line (comm line)/strength member to the diver. -
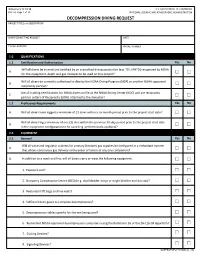
NOAA Form 57-03-28 Decompression Diving Request
NOAA Form 57-03-28 U.S. DEPARTMENT OF COMMERCE ( 03-15) Page 1 of 10 NATIONAL OCEANIC AND ATMOSPHERIC ADMINISTRATION DECOMPRESSION DIVING REQUEST PROJECT TITLE and DESCRIPTION DIVER SUBMITTING REQUEST DATE E-MAIL ADDRESS PHONE NUMBER 1.0 QUALIFICATIONS 1.1 Certification and Authorization Yes No Will all divers be trained and certified by an accredited diving association (e.g. TDI, IANTD) recognized by NOAA A. for the equipment, depth and gas mixtures to be used on this project? Will all divers be currently authorized to dive by the NOAA Diving Program (NDP) or another NOAA-approved B. reciprocity partner? Are all training certifications for NOAA divers on file at the NOAA Diving Center (NDC) and are reciprocity C. partner Letters of Reciprocity (LORs) attached to the dive plan? 1.2 Proficiency Requirements Yes No A. Will all divers have logged a minimum of 12 dives within a six month period prior to the project start date? Will all divers log a minimum of one (1) dive within the previous 30-day period prior to the project start date B. in the equipment configuration to be used (e.g. perform work-up dives)? 2.0 EQUIPMENT 2.1 General Yes No Will all valve and regulator systems for primary (bottom) gas supplies be configured in a redundant manner A. that allows continuous gas delivery in the event of failure of any one component? B. In addition to a mask and fins, will all divers carry or wear the following equipment: 1. Exposure suit? 2. Buoyancy Compensator Device (BCD) (e.g. -

Physiological Constraints and Energetic Costs of Diving Behaviour in Marine Mammals: a Review of Studies Using Trained Steller Sea Lions Diving in the Open Ocean
J Comp Physiol B (2017) 187:29–50 DOI 10.1007/s00360-016-1035-8 REVIEW Physiological constraints and energetic costs of diving behaviour in marine mammals: a review of studies using trained Steller sea lions diving in the open ocean David A. S. Rosen1 · Allyson G. Hindle1 · Carling D. Gerlinsky1 · Elizabeth Goundie1 · Gordon D. Hastie1 · Beth L. Volpov1 · Andrew W. Trites1 Received: 17 February 2016 / Revised: 26 August 2016 / Accepted: 13 September 2016 / Published online: 29 September 2016 © Springer-Verlag Berlin Heidelberg 2016 Abstract Marine mammals are characterized as having basis of diving behaviour further our understanding of the physiological specializations that maximize the use of oxy- potential scope for behavioural responses of marine mam- gen stores to prolong time spent under water. However, it mals to environmental changes, the energetic significance has been difficult to undertake the requisite controlled stud- of these adjustments, and the consequences of approaching ies to determine the physiological limitations and trade-offs physiological limits. that marine mammals face while diving in the wild under varying environmental and nutritional conditions. For the Keywords Diving physiology · Steller sea lions · past decade, Steller sea lions (Eumetopias jubatus) trained Metabolism · Foraging to swim and dive in the open ocean away from the physi- cal confines of pools participated in studies that investi- gated the interactions between diving behaviour, energetic The need to study diving metabolism costs, physiological constraints, and prey availability. Many of these studies measured the cost of diving to understand Marine mammals are well known for being able to remain how it varies with behaviour and environmental and physi- submerged for extended durations.