Review of Species Selected on the Basis of the Analysis of the European Union and Candidate Countries’ Annual Reports to CITES 2009
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biotechnologies from Marine Bivalves
Nutrient Extraction Through Bivalves Petersen, Jens Kjerulf; Holmer, Marianne; Termansen, Mette; Hasler, Berit Published in: Goods and Services of Marine Bivalves DOI: 10.1007/978-3-319-96776-9_10 Publication date: 2019 Document version Publisher's PDF, also known as Version of record Citation for published version (APA): Petersen, J. K., Holmer, M., Termansen, M., & Hasler, B. (2019). Nutrient Extraction Through Bivalves. In A. C. Smaal, J. G. Ferreira, J. Grant, J. K. Petersen, & Ø. Strand (Eds.), Goods and Services of Marine Bivalves (pp. 179-208). Springer. https://doi.org/10.1007/978-3-319-96776-9_10 Download date: 05. okt.. 2021 Aad C. Smaal · Joao G. Ferreira · Jon Grant Jens K. Petersen · Øivind Strand Editors Goods and Services of Marine Bivalves Goods and Services of Marine Bivalves Just the pearl II, by Frank van Driel, fine art photography (www.frankvandriel.com), with painted oyster shells of www.zeeuwsblauw.nl Aad C. Smaal • Joao G. Ferreira • Jon Grant Jens K. Petersen • Øivind Strand Editors Goods and Services of Marine Bivalves Editors Aad C. Smaal Joao G. Ferreira Wageningen Marine Research and Universidade Nova de Lisboa Aquaculture and Fisheries group Monte de Caparica, Portugal Wageningen University and Research Yerseke, The Netherlands Jens K. Petersen Technical University of Denmark Jon Grant Nykøbing Mors, Denmark Department of Oceanography Dalhousie University Halifax, Nova Scotia, Canada Øivind Strand Institute of Marine Research Bergen, Norway ISBN 978-3-319-96775-2 ISBN 978-3-319-96776-9 (eBook) https://doi.org/10.1007/978-3-319-96776-9 Library of Congress Control Number: 2018951896 © The Editor(s) (if applicable) and The Author(s) 2019 , corrected publication 2019. -

Remnants of the Early Dutch in Guyana 1616-1815 Nova Zeelandia (New Zeeland
Remnants Of The Early Dutch in Guyana 1616-1815 By Dmitri Allicock Coat of arms -Flag of the Dutch West Indian Company- 1798 Map of Essequibo and Demerara Nova Zeelandia (New Zeeland} Guyana is the only English-speaking country in South America, but English has been the official language for less than half the time Europeans occupied the country. The Dutch language was the main medium of communication for 232 years, from the time a group of Dutchmen sailed up the Pomeroon River and settled there, to 1812 when English replaced Dutch as the language used in the Court of Policy (Parliament). To this day, hundreds of villages have retained their original Dutch names like Uitvlugt, Vergenoegen and Zeeburg. Some present-day Guyanese have names like Westmaas, Van Lange and Meertens. No Guyanese citizen or visitor can escape visible and other reminders of our Dutch predecessors. The ruins of a brick fort can still be seen on a little island where the Essequibo, Mazaruni and Cuyuni rivers meet. The original fort was a wooden structure built around 1600 by some Dutch traders who called it Kyk-over-al or "See-over-all" because it provided a commanding view of the three rivers. From 1627 the fort was controlled by the Dutch West India Company, a Holland-based organization which was vested with the power to establish colonies and which monopolized Dutch trade in the New World. The Company appointed Adrianetz Groenewegel as its first Commander to administer Kyk-over-al. The wooden fort was replaced in the 1630s by a brick structure which also served as an administrative centre. -

Competent Persons Report for Certain Assets in Offshore Guyana
Competent Persons Report for Certain Assets in Offshore Guyana Date of this Report: September 11, 2018 Prepared for: ECO (Atlantic) Oil and Gas Ltd Prepared By: Phone: 1-303-443-2209, Fax: 1-303-443-3156 E-mail: [email protected] Competent Persons Report for Certain Assets in Offshore Guyana Date of this Report: September 11, 2018 Prepared for: ECO (Atlantic) Oil and Gas Ltd Prepared By: Kevin S. Weller Registered Petroleum Engineer State of Colorado #34214 Phone: 1-303-443-2209, Fax: 1-303-443-3156 E-mail: [email protected] m 1. EXECUTIVE SUMMARY The report addresses the ECO (Atlantic) Oil and Gas Ltd (“ECO Atlantic”, “ECO”, “The Company”) exploratory oil and gas assets in offshore Guyana. The assets owned by ECO Atlantic are summarized in Table 1-1. Table 1-1 Summary of Assets owned by ECO (Atlantic) Oil and Gas Ltd Working License Water Asset Operator Interest Status Expiry Date Area Depth, (%) (km2)1 meters January 70 to Orinduik Block Tullow 40.0 Exploration 1,800 2026 1,250 Based on probabilistic estimates, the Gross (100%) and Net (40%) Unrisked Prospective Resources for the Orinduik Block of Guyana in millions of barrels of oil equivalent (MMBOE6) are listed below in Table 1-2. This is based on a 6:1 gas to oil equivalency. The Gross Unrisked Prospective Resources are presented in Table 1-3 and the Net Unrisked Prospective Resources for the Orinduik Block of Guyana are listed in Table 1-4 below. Table 1-2 Gross and Net Barrels of Oil Equivalent Unrisked Prospective Resources Gross Prospective Oil Net Prospective Oil -
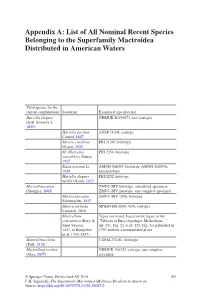
List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters
Appendix A: List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters Valid species (in the current combination) Synonym Examined type material Harvella elegans NHMUK 20190673, two syntypes (G.B. Sowerby I, 1825) Harvella pacifica ANSP 51308, syntype Conrad, 1867 Mactra estrellana PRI 21265, holotype Olsson, 1922 M. (Harvella) PRI 2354, holotype sanctiblasii Maury, 1925 Raeta maxima Li, AMNH 268093, lectotype; AMNH 268093a, 1930 paralectotype Harvella elegans PRI 2252, holotype tucilla Olsson, 1932 Mactrellona alata ZMUC-BIV, holotype, articulated specimen; (Spengler, 1802) ZMUC-BIV, paratype, one complete specimen Mactra laevigata ZMUC-BIV 1036, holotype Schumacher, 1817 Mactra carinata MNHN-IM-2000-7038, syntypes Lamarck, 1818 Mactrellona Types not found, based on the figure of the concentrica (Bory de “Tableau of Encyclopedique Methodique…” Saint Vincent, (pl. 251, Fig. 2a, b, pl. 252, Fig. 2c) published in 1827, in Bruguière 1797 without a nomenclatorial act et al. 1791–1827) Mactrellona clisia USNM 271481, holotype (Dall, 1915) Mactrellona exoleta NHMUK 196327, syntype, one complete (Gray, 1837) specimen © Springer Nature Switzerland AG 2019 103 J. H. Signorelli, The Superfamily Mactroidea (Mollusca:Bivalvia) in American Waters, https://doi.org/10.1007/978-3-030-29097-9 104 Appendix A: List of All Nominal Recent Species Belonging to the Superfamily… Valid species (in the current combination) Synonym Examined type material Lutraria ventricosa MCZ 169451, holotype; MCZ 169452, paratype; -

The Orchidaceae of Primitiae Florae Essequeboensis (1818)
LANKESTERIANA 18(3): 239–242. 2018. doi: https://doi.org/10.15517/lank.v18i3.35625 THE ORCHIDACEAE OF PRIMITIAE FLORAE ESSEQUEBOENSIS (1818) CARLOS OSSENBACH Orquideario 25 de Mayo, San José, Costa Rica and Lankester Botanical Garden, University of Costa Rica [email protected] ABSTRACT. The German botanist and Professor at the University of Göttingen, Georg Friedrich Wilhelm Meyer (1782–1856), studied the plants collected in the Dutch colony of Essequibo by Ernst Carl Rodschied and those kept in the herbarium of Professor Franz Karl Mertens, which he had received from a Dutch colonist during the early 1800s. On that basis, he published in 1818 his work Primitiae Florae Essequeboensis, describing 344 species of plants. Among them there are five species of orchids, two of which were new to science. KEY WORDS: Essequibo, Georg Friedrich Wilhelm Meyer, Guiana, Orchidaceae Essequibo (or Essequebo in Dutch) was a Dutch on the east, on the eastern border of the Spanish colony on the northern coast of South America from General Captaincy of Venezuela in the Guiana region. 1616 to 1814 (Fig. 1). It was founded between the It formed a part of the settlements that are known under Essequibo River on the west and the Demerara River the collective name of Dutch Guiana. Essequibo’s FIGURE 1. Carte generale et particuliere de la colonie d’Essequebe & Demerarie située dans la Guiane en Amérique. Brave & Wouter (1798). Received 3 July 2018; accepted for publication 11 December 2018. First published online: 17 December 2018. Licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Costa Rica License 240 LANKESTERIANA FIGURE 3. -

Caribbean Sub-Region 35 177K 141K 25K
Caribbean Sub-Region Situation Report - March 2019 Caribbean Sub-Regional Refugee & Migrants Response Plan - Summary Figures Dominican Republic Trinidad and Guyana Aruba Curaçao Tobago 35 177K 141K 25K 35M Appealing Refugees & Refugees & Target Host Financial Partners Migrants in Need Migrants Targeted Community Requirements In recent years, five countries in the sub-region have been hosting growing numbers of refugees and migrants from Venezuela: Aruba, Curaçao, the Dominican Republic, Guyana, and Trinidad and Tobago. It is estimated that around 146,9001 have arrived by air, land, and sea to date, including some returnees in Guyana. Through the 2019 Regional Refugee and Migrants Response Plan (RRMRP), partners have identified the priority needs for both refugees/migrants, and host communities. These are: protection, shelter, access to food, adequate nutrition and health care, including psychosocial support, as well as the provision of livelihoods, and self-reliance opportunities. The RRMRP outlines the intended interventions to address these priority needs and their related financial requirements for the sub-region, in support to the response of national governments. Through the inter-agency coordination platforms (Refugee and Migrants Working Groups) that are being established at the sub-regional and national level, United Nations (UN) agencies, Non- Governmental Organizations (NGOs), and civil society aim to meet the emergency assistance, protection, and integration needs of refugees and migrants from Venezuela, in complementarity and support to existing government’s responses. KEY FIGURES (as of March 2019) Over 146,900 Venezuelans arrived in 11,855 Registered Venezuelan asylum seekers the Caribbean sub-region since 2015.1 in the Caribbean sub-region since 2015.2 No. -

The West Indian Web Improvising Colonial Survival in Essequibo and Demerara, 1750-1800
The West Indian Web Improvising colonial survival in Essequibo and Demerara, 1750-1800 Bram Hoonhout Thesis submitted for assessment with a view to obtaining the degree of Doctor of History and Civilization of the European University Institute Florence, 22 February 2017 European University Institute Department of History and Civilization The West Indian Web Improvising colonial survival in Essequibo and Demerara, 1750-1800 Bram Hoonhout Thesis submitted for assessment with a view to obtaining the degree of Doctor of History and Civilization of the European University Institute Examining Board Prof. dr. Jorge Flores (EUI) Prof. dr. Regina Grafe (EUI) Prof. dr. Cátia Antunes (Leiden University) Prof. dr. Gert Oostindie, KITLV/Royal Netherlands Institute of Southeast Asian and Caribbean Studies © Bram Hoonhout, 2017 No part of this thesis may be copied, reproduced or transmitted without prior permission of the author Researcher declaration to accompany the submission of written work Department of History and Civilization - Doctoral Programme I Bram Hoonhoutcertify that I am the author of the work The West Indian web. Improvising colonial survival in Essequibo and Demerara, 1750-1800 I have presented for examination for the Ph.D. at the European University Institute. I also certify that this is solely my own original work, other than where I have clearly indicated, in this declaration and in the thesis, that it is the work of others. I warrant that I have obtained all the permissions required for using any material from other copyrighted publications. I certify that this work complies with the Code of Ethics in Academic Research issued by the European University Institute (IUE 332/2/10 (CA 297). -

Preliminary Satellite- Derived Flood Assessment
18 June 2021 PRELIMINARY SATELLITE- DERIVED FLOOD ASSESSMENT Along the Essequibo and Demerara rivers in Right Bank Essequibo Sub-Region, Region 10- Upper Demerara-Berbice, Guyana Status: Inundated structures near Essequibo river and along Demerara rivers observed Further action(s): continue monitoring GUYANA AREA OF INTEREST (AOI) 18 June 2021 REGION FLOODS OVER GUYANA N 120 km Region 1 Region 2 Region 3 Region 4 Region 7 Region 5 AOI Region 10 Region 8 Legend Satellite detected water Region boundary as of 16 and17 June 2021 International boundary River Region 6 Satellite detected water as of 16 and 17 June 2021 [Joint ABI/VIIRS] Region 9 Cloud mask Area of interest Background: ESRI Basemap 3 N Right Bank Essequibo Sub-Region, Region 10- Upper Demerara-Berbice 3 km AOI 2 AOI Torani/Bul Linden city letwood Region 10 AOI 1 Right Bank Essequibo Rockstone town AOI 3 Worldview-2 / 15 Jun 2021 Image center: AOI 1 Right Bank Essequibo Sub-Region, 58°32'21.815"W Region 10- Upper Demerara-Berbice 5°59'28.371"N Inundated structures near Essequibo river observed BEFORE AFTER Inundated structures N 60 m Worldview-2 / 16 Dec 2017 Worldview-2 / 15 Jun 2021 5 AOI 2 Image center: AOI 2-1 Right Bank Essequibo Sub-Region, 58°17'35.647"W Region 10- Upper Demerara-Berbice 6°1'32.678"N No floods observed BEFORE AFTER N 150 m Worldview-2 / 26 Aug 2020 Worldview-2 / 15 Jun 2021 6 AOI 2 Image center: AOI 2-2 Linden city, Right Bank Essequibo Sub-Region, 58°18'57.245"W Region 10- Upper Demerara-Berbice 6°0'59.099"N No floods observed BEFORE AFTER Linden city Linden -

REPUBLIC of GUYANA V. REPUBLIC of SURINAME
ARBITRATION UNDER ANNEX VII OF THE UNITED NATIONS CONVENTION ON THE LAW OF THE SEA REPUBLIC OF GUYANA v. REPUBLIC OF SURINAME MEMORIAL OF THE REPUBLIC OF GUYANA VOLUME I 22 FEBRUARY 2005 Memorial of Guyana MEMORIAL OF GUYANA PART I 2 Memorial of Guyana TABLE OF CONTENTS VOLUME I Page CHAPTER 1 - INTRODUCTION..........................................................................................1 I. Reasons for the Institution of Proceedings Against Suriname..............................1 II. Guyana’s Approach to the Presentation of the Case.............................................3 III. Structure of the Memorial.....................................................................................3 CHAPTER 2 - GEOGRAPHY AND EARLY HISTORY ...................................................7 I. Geography.............................................................................................................7 II. Early History.......................................................................................................10 CHAPTER 3 - EFFORTS OF THE COLONIAL POWERS TO SETTLE THE BOUNDARY BETWEEN BRITISH GUIANA AND SURINAME: 1929 TO 1966........13 I. The Fixing of the Northern Land Boundary Terminus between British Guiana and Suriname: 1936................................................................................14 II. The First Attempt To Fix a Maritime Boundary in the Territorial Sea: 1936 ....18 III. The Draft Treaty To Settle the Entire Boundary: 1939 ......................................20 IV. Unsuccessful Post-World -

Ask Most Casual Students of History When the Time of Colonialism Came to an End
ABSTRACT Guiana and the Shadows of Empire: Colonial and Cultural Negotiations at the Edge of the World Joshua R. Hyles, M.A. Mentor: Keith A. Francis, Ph.D. Nowhere in the world can objective study of colonialism and its effects be more fruitful than in the Guianas, the region of three small states in northeastern South America. The purpose of this thesis is threefold. First, the history of these three Guianas, now known as Guyana, French Guiana, and Suriname, is considered briefly, emphasizing their similarities and regional homogeny when compared to other areas. Second, the study considers the administrative policies of each of the country’s colonizers, Britain, France, and the Netherlands, over the period from settlement to independence. Last, the thesis concentrates on current political and cultural situations in each country, linking these developments to the policies of imperial administrators in the previous decades. By doing so, this thesis hopes to show how an area that should have developed as a single polity could become a region of three very distinct cultures through the altering effects of colonialism. Guiana and the Shadows of Empire: Colonial and Cultural Negotiations at the Edge of the World by Joshua R. Hyles, A.S., B.A. A Thesis Approved by the Department of History ___________________________________ Jeffrey S. Hamilton, Ph.D., Chairperson Submitted to the Graduate Faculty of Baylor University in Partial Fulfillment of the Requirements for the Degree of Master of Arts Approved by the Thesis Committee ___________________________________ Keith A. Francis, Ph.D., Chairperson ___________________________________ David L. Longfellow, Ph.D. ___________________________________ Thomas A. -

Supplement – December 2017 – Survey of the Literature on Recent
A Malacological Journal ISSN 1565-1916 No. 36 - SUPPLEMENT DECEMBER 2017 2 SURVEY OF THE LITERATURE ON RECENT SHELLS FROM THE RED SEA (third enlarged and revised edition) L.J. van Gemert* Summary This literature survey lists approximately 3,050 references. Shells are being considered here as the shell bearing molluscs of the Gastropoda, Bivalvia and Scaphopoda. The area does not only comprise the Red Sea, but also the Gulf of Aden, Somalia and the Suez Canal, including the Lessepsian species in the Mediterranean Sea. Literature on fossils shells, particularly those from the Holocene, Pleistocene and Pliocene, is listed too. Introduction My interest in recent shells from the Red Sea dates from about 1996. Since then, I have been, now and then, trying to obtain information on this subject. Some years ago I decide to stop gathering data in a haphazard way and to do it more properly. This resulted in a first survey of approximately 1,420 and a second one of 2,025 references (van Gemert, 2010 & 2011). Since then, this survey has again been enlarged and revised and a number of errors have been corrected. It contains now approximately 3,050 references. Scope In principle every publication in which molluscs are reported to live or have lived in the Red Sea should be listed in the survey. This means that besides primary literature, i.e. articles in which researchers are reporting their finds for the first time, secondary and tertiary literature, i.e. reviews, monographs, books, etc are to be included too. These publications were written not only by a wide range of authors ranging from amateur shell collectors to professional malacologists but also people interested in the field of archaeology, geology, etc. -

British Guiana – British Empire Exhibition, Wembley 1924
British Guiana – British Empire Exhibition, Wembley 1924 BRITISH GUIANA BRITISH EMPIRE EXHIBITION WEMBLEY 1924 CONTENTS 1 British Guiana – British Empire Exhibition, Wembley 1924 INTRODUCTION GEOGRAPHY KAIETEUR FALLS POPULATION Aborigines Immigration Centres of Population FAUNA FLORA TIMBER AND FOREST INDUSTRIES SUGAR, R ICE AND AGRICULTURAL INDUSTRIES MINING INDUSTRIES COMMERCE AND SHIPPING Communications OPPORTUNITIES FOR CAPITAL 2 British Guiana – British Empire Exhibition, Wembley 1924 LIST OF ILLUSTRATIONS Note: Click here to view these photos Kaieteur Falls, Potaro River A Locust Tree (Hymencea courbaril) A Road in the Interior, 150 miles Heading out Sugar Canes to Punts from Georgetown Potato River, on the way to Steam Hoist Lifting Canes at a Kaieteur Falls Sugar Factory Brink of Kaieteur Falls Loading the Cut Cane into Punts on a Sugar Estate In the Gorge below Kaieteur Falls; Ploughing a Rice Field looking down river Amatuk Rest House, Potaro River, Harvesting the Rice on the way to Kaieteur Falls Gold Digging Camp at foot of A Coconut Plantation Eagle Mountain Wismar, 65 miles up Demerara Indian Girl Spinning Cotton River Kanaka Mountains and Interior The Rupununi Cattle Trail, Bridge Savannas Over a Creek Aboriginal Indians Travelling by Steer Bred on Rupununi Savanna Corial Indian Shooting Fish Rupununi River, looking North to the Kanaka Mountains The Public Buildings, Georgetown Alluvial Gold Mining Main Street, Georgetown, and the Cattle on the Rupununi Savannas War Memorial 3 British Guiana – British Empire Exhibition,