Apo-Nid110846.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biotechnologies from Marine Bivalves
Nutrient Extraction Through Bivalves Petersen, Jens Kjerulf; Holmer, Marianne; Termansen, Mette; Hasler, Berit Published in: Goods and Services of Marine Bivalves DOI: 10.1007/978-3-319-96776-9_10 Publication date: 2019 Document version Publisher's PDF, also known as Version of record Citation for published version (APA): Petersen, J. K., Holmer, M., Termansen, M., & Hasler, B. (2019). Nutrient Extraction Through Bivalves. In A. C. Smaal, J. G. Ferreira, J. Grant, J. K. Petersen, & Ø. Strand (Eds.), Goods and Services of Marine Bivalves (pp. 179-208). Springer. https://doi.org/10.1007/978-3-319-96776-9_10 Download date: 05. okt.. 2021 Aad C. Smaal · Joao G. Ferreira · Jon Grant Jens K. Petersen · Øivind Strand Editors Goods and Services of Marine Bivalves Goods and Services of Marine Bivalves Just the pearl II, by Frank van Driel, fine art photography (www.frankvandriel.com), with painted oyster shells of www.zeeuwsblauw.nl Aad C. Smaal • Joao G. Ferreira • Jon Grant Jens K. Petersen • Øivind Strand Editors Goods and Services of Marine Bivalves Editors Aad C. Smaal Joao G. Ferreira Wageningen Marine Research and Universidade Nova de Lisboa Aquaculture and Fisheries group Monte de Caparica, Portugal Wageningen University and Research Yerseke, The Netherlands Jens K. Petersen Technical University of Denmark Jon Grant Nykøbing Mors, Denmark Department of Oceanography Dalhousie University Halifax, Nova Scotia, Canada Øivind Strand Institute of Marine Research Bergen, Norway ISBN 978-3-319-96775-2 ISBN 978-3-319-96776-9 (eBook) https://doi.org/10.1007/978-3-319-96776-9 Library of Congress Control Number: 2018951896 © The Editor(s) (if applicable) and The Author(s) 2019 , corrected publication 2019. -

Profile of Nutrition and Hazards of Om-Elkholool (Donax Trunculus) and Gandofly (Ruditapes Decussatus) Clams from Alexandria, Egypt
International Journal For Research In Agricultural And Food Science ISSN: 2208-2719 Profile of Nutrition and Hazards of Om-Elkholool (Donax Trunculus) and Gandofly (Ruditapes Decussatus) Clams From Alexandria, Egypt Sherief Mohammed Sayed Abd-Allah Assistant Professor, Department of Food Hygiene "Meat Hygiene", Faculty of Veterinary Medicine, Assiut University, Assiut 71526, Egypt Email:[email protected] ABSTRACT Clams are delicate nutritious food; however they can harbor potential health hazards. The current work aimed to investigate and compare some of the nutritive criteria and hazards of Om-Elkholool (Donax trunculus) and Gandofly (Ruditapes decussatus) clams sold at Alexandria, Egypt. A total of 46 samples (22 of Om-Elkholool and 24 of Gandofly) were randomly collected from fish retailers during summer of 2017. Samples were analyzed for proximate composition (dry matter, moisture, protein, fat, and ash %). The carbohydrates and energy content was calculated. The count of coliforms, fecal coliforms, E. coli and Cl. perfringenes (MPN/g) was determined. Concentration (mg/kg) of lead and cadmium in 10 randomly selected samples of each type were estimated. The dry matter, moisture, protein, fat, ash and carbohydrates percentages mean values for Om-Elkholool “Om” samples were 30.37±0.22, 69.60±0.21, 8.49±0.14, 1.29±0.03, 18.63±0.09, and 1.99±11, respectively, while for Gandofly “Gd” samples were 16.81±0.21, 83.28±0.2, 8.69±0.13, 1.22±0.03, 3.43±0.09, and 3.37±10, respectively. The gross energy content (Kcal/100g) mean value was 53.55±0.88 for Om and 59.24±0.85 for Gd. -

(Venerupis Decussata, Linnaeus, 1758), from the Tunisian Coast: Trophic Links
GRASAS Y ACEITES 70 (1) January–March 2019, e289 ISSN-L: 0017-3495 https://doi.org/10.3989/gya.0580181 Geographic variation in fatty acid composition and food source of the commercial clam (Venerupis decussata, Linnaeus, 1758), from the Tunisian Coast: Trophic links S. Bejaouia,*, D. Boussoufaa¥, K. Telahiguea¥, I. Chetouia, F. Ghribia, I. Rabeha and M. El Cafsia aUnit of Physiology and Aquatic Environment, Biology Department, Faculty of Science of Tunis, University of Tunis El Manar, 2092, Tunis, Tunisia. ¥ These authors contribute equally to this work *Corresponding author: [email protected] Submitted: 25 May 2018; Accepted: 26 July 2018 SUMMARY: Lake and coastal Tunisian areas are rich biodiversity habitats, although little information is available about the distribution of food sources for the inhabitant species. In this study, a fatty acid analysis was used to study the trophic ecology of Venerupis decussatac ommunities from 10 sites located along the Tunisian Coast. The richest population in fatty acids was found in S4 followed by S5 and S8, while that of S1, S3 and S10 were the least rich. Results from multivariate analysis confirmed the ecological position of the studied population based on their fatty acid composition. Our results divided the ten studied populations into three similar groups according to their eco- logical and geographical positions in relation to environmental parameters and food and trophic links. A principal component analysis revealed that diatoms and dinoflagellates were the predominate diets in all the sampling stations. Bacteria and urban discharge dominated the dietary source of clams from S10 and S9. Zooplankton were the pre- ferred diet of V. -

The Evolution of the Molluscan Biota of Sabaudia Lake: a Matter of Human History
SCIENTIA MARINA 77(4) December 2013, 649-662, Barcelona (Spain) ISSN: 0214-8358 doi: 10.3989/scimar.03858.05M The evolution of the molluscan biota of Sabaudia Lake: a matter of human history ARMANDO MACALI 1, ANXO CONDE 2,3, CARLO SMRIGLIO 1, PAOLO MARIOTTINI 1 and FABIO CROCETTA 4 1 Dipartimento di Biologia, Università Roma Tre, Viale Marconi 446, I-00146 Roma, Italy. 2 IBB-Institute for Biotechnology and Bioengineering, Center for Biological and Chemical Engineering, Instituto Superior Técnico (IST), 1049-001, Lisbon, Portugal. 3 Departamento de Ecoloxía e Bioloxía Animal, Universidade de Vigo, Lagoas-Marcosende, Vigo E-36310, Spain. 4 Stazione Zoologica Anton Dohrn, Villa Comunale, I-80121 Napoli, Italy. E-mail: [email protected] SUMMARY: The evolution of the molluscan biota in Sabaudia Lake (Italy, central Tyrrhenian Sea) in the last century is hereby traced on the basis of bibliography, museum type materials, and field samplings carried out from April 2009 to Sep- tember 2011. Biological assessments revealed clearly distinct phases, elucidating the definitive shift of this human-induced coastal lake from a freshwater to a marine-influenced lagoon ecosystem. Records of marine subfossil taxa suggest that previous accommodations to these environmental features have already occurred in the past, in agreement with historical evidence. Faunal and ecological insights are offered for its current malacofauna, and special emphasis is given to alien spe- cies. Within this framework, Mytilodonta Coen, 1936, Mytilodonta paulae Coen, 1936 and Rissoa paulae Coen in Brunelli and Cannicci, 1940 are also considered new synonyms of Mytilaster Monterosato, 1884, Mytilaster marioni (Locard, 1889) and Rissoa membranacea (J. -

For Breeding of Venerupis Decussata (Linnaeus, 1758) Juveniles in a Coastal Lagoon in Sardinia (Italy) G
Transitional Waters Bulletin TWB, Transit. Waters Bull. 7 (2013), n. 2, 53-61 ISSN 1825-229X, DOI 10.1285/i1825229Xv7n2p53 http://siba-ese.unisalento.it The floating upwelling system (FLUPSY) for breeding of Venerupis decussata (Linnaeus, 1758) juveniles in a coastal lagoon in Sardinia (Italy) G. Chessa*, S. Serra, S. Saba, S. Manca, F. Chessa, M. Trentadue, N. Fois AGRIS Sardegna, Dipartimento per la Ricerca nelle Produzioni Animali, RESEARCH ARTICLE Servizio Risorse Ittiche, Località Bonassai S.S. 291 km 18.600, 07040 Olmedo (SS), Italy. *Corresponding author: Phone: +39 079 2842371; Fax: +39 079 389450; E-mail address:[email protected] Abstract 1 - In recent years, interest in farming the grooved carpet shell Venerupis decussata (Linnaeus, 1758) in Sardinian lagoons has greatly increased due to a decrease in clam populations. Aquaculturists around the world have developed a variety of different clam farming systems, notably for the Manila clam, Venerupis philippinarum (Adams & Reeve, 1850). 2 - The aim was to test the aquaculture of V. decussata using the FLoating UPwelling SYstem (FLUPSY) to evaluate their growth rate over two seasons: spring 2011 (from 10 March, 2011, to 21 May, 2011), and autumn 2011 (from 18 October, 2011, to 28 December, 2011). The study was carried out in the Tortolì coastal lagoon, Eastern Sardinia (Italy; latitude 39°56’ N, longitude 9°41’ E). 3 - V. decussata juveniles with mean shell lengths of 10.88±0.91 mm in spring and 8.95 ± 0.75 mm in autumn, mean shell thicknesses of 5.21±0.54 mm in spring and 3.36 ± 0.27 mm in autumn, and mean total weight of 0.33±0.09 g in spring and 0.1 2± 0.03 g in autumn, were bred following FLUPSY. -

Ocean Acidification Impact on the Grooved Carpet Shell Clam (Ruditapes Decussatus)
Egyptian Journal of Aquatic Biology & Fisheries Zoology Department, Faculty of Science, Ain Shams University, Cairo, Egypt. ISSN 1110 – 6131 Vol. 23(5): 169 - 182 (2019) www.ejabf.journals.ekb.eg Ocean acidification impact on the grooved carpet shell clam (Ruditapes decussatus) Merna E. Awad1, Nayrah A. Shaltout2, Fedekar F. Madkour1, Mohamed A. Abu El-Regal1, Heba S. El-Sayed*3, Eman El-Wazzan3 1- Marine Science Department, Faculty of Science, Port Said University, Port Said, Egypt 2- Marine Chemistry Department, National Institute of Oceanography and Fisheries, Alexandria, Egypt 3- Aquaculture Division, National Institute of Oceanography and Fisheries, Alexandria, Egypt * Corresponding author : [email protected] ARTICLE INFO ABSTRACT Article History: The grooved carpet shell clam (Ruditapes decussatus) is one of the most Received: May 2, 2019 economicallyimportant mollusks inhabiting Mediterranean lagoons and Accepted: Nov. 28, 2019 sandy beaches both from fisheries and aquaculture. The present study aims Online: Dec. 2019 to study the impact of different levels of acidification on this calcifying _______________ organism. Juvenile clams (avg. Shell Length, SL= 23.22 ± 0.84 mm) were incubated in CO enriched seawater at four different CO concentrations Keywords: 2 2 [420 ppm (ambient control), 550 ppm, 750 ppm and 1050 ppm] representing Ocean acidification projected atmospheric CO concentration scenarios for the year 2100 by grooved carpet clam 2 IPCC. The studied biological parameters showed slight decrease with Ruditapes decussatus increasing pCO . However, differences were not significant. Standard length Calcifying organism 2 decreased as pCO concentration increased, with a maximum average Biological impact 2 decrease of (-0.12) recorded at 750 ppm as compared to the control group. -

Marlin Marine Information Network Information on the Species and Habitats Around the Coasts and Sea of the British Isles
MarLIN Marine Information Network Information on the species and habitats around the coasts and sea of the British Isles Pullet carpet shell (Venerupis corrugata) MarLIN – Marine Life Information Network Marine Evidence–based Sensitivity Assessment (MarESA) Review Will Rayment 2007-08-13 A report from: The Marine Life Information Network, Marine Biological Association of the United Kingdom. Please note. This MarESA report is a dated version of the online review. Please refer to the website for the most up-to-date version [https://www.marlin.ac.uk/species/detail/1558]. All terms and the MarESA methodology are outlined on the website (https://www.marlin.ac.uk) This review can be cited as: Rayment, W.J. 2007. Venerupis corrugata Pullet carpet shell. In Tyler-Walters H. and Hiscock K. (eds) Marine Life Information Network: Biology and Sensitivity Key Information Reviews, [on-line]. Plymouth: Marine Biological Association of the United Kingdom. DOI https://dx.doi.org/10.17031/marlinsp.1558.1 The information (TEXT ONLY) provided by the Marine Life Information Network (MarLIN) is licensed under a Creative Commons Attribution-Non-Commercial-Share Alike 2.0 UK: England & Wales License. Note that images and other media featured on this page are each governed by their own terms and conditions and they may or may not be available for reuse. Permissions beyond the scope of this license are available here. Based on a work at www.marlin.ac.uk (page left blank) Date: 2007-08-13 Pullet carpet shell (Venerupis corrugata) - Marine Life Information Network See online review for distribution map Distribution data supplied by the Ocean Biogeographic Information System (OBIS). -
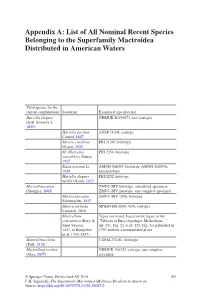
List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters
Appendix A: List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters Valid species (in the current combination) Synonym Examined type material Harvella elegans NHMUK 20190673, two syntypes (G.B. Sowerby I, 1825) Harvella pacifica ANSP 51308, syntype Conrad, 1867 Mactra estrellana PRI 21265, holotype Olsson, 1922 M. (Harvella) PRI 2354, holotype sanctiblasii Maury, 1925 Raeta maxima Li, AMNH 268093, lectotype; AMNH 268093a, 1930 paralectotype Harvella elegans PRI 2252, holotype tucilla Olsson, 1932 Mactrellona alata ZMUC-BIV, holotype, articulated specimen; (Spengler, 1802) ZMUC-BIV, paratype, one complete specimen Mactra laevigata ZMUC-BIV 1036, holotype Schumacher, 1817 Mactra carinata MNHN-IM-2000-7038, syntypes Lamarck, 1818 Mactrellona Types not found, based on the figure of the concentrica (Bory de “Tableau of Encyclopedique Methodique…” Saint Vincent, (pl. 251, Fig. 2a, b, pl. 252, Fig. 2c) published in 1827, in Bruguière 1797 without a nomenclatorial act et al. 1791–1827) Mactrellona clisia USNM 271481, holotype (Dall, 1915) Mactrellona exoleta NHMUK 196327, syntype, one complete (Gray, 1837) specimen © Springer Nature Switzerland AG 2019 103 J. H. Signorelli, The Superfamily Mactroidea (Mollusca:Bivalvia) in American Waters, https://doi.org/10.1007/978-3-030-29097-9 104 Appendix A: List of All Nominal Recent Species Belonging to the Superfamily… Valid species (in the current combination) Synonym Examined type material Lutraria ventricosa MCZ 169451, holotype; MCZ 169452, paratype; -

Portadas 25 (1)
© Sociedad Española de Malacología Iberus, 32 (1): 65-85, 2014 Nomenclatural notes on some European marine bivalve species Apuntes nomenclaturales sobre algunas especies de bivalvos de Europa Rudo von COSEL*, Serge GOFAS** & Jean-Maurice POUTIERS* Recibido el 6-XI-2013. Aceptado el 17-I-2014 ABSTRACT Some nomenclatural issues affecting European species are discussed. The following taxa are treated under ICZN Art. 23.9, with the required references provided: - Mytilus variabilis Krauss, 1848 (currently Brachidontes variabilis (Krauss, 1848)) is declared nomen protectum against the senior homonym Mytilus variabilis Fischer von Waldheim, 1807, declared nomen oblitum. The still earlier name Brachidontes ustulatus (Lamarck, 1819), currently used as the valid name for a native species of Western Aus- tralia, should take precedence over B. variabilis (Krauss, 1848) were it demonstrated that it is the same biological species, but in the current state of knowledge it is proposed to keep them separate. - Modiola nigra Gray, 1824 (currently Musculus niger (Gray, 1824)) is declared nomen protectum against the senior synonym Mytilus discors svecicus Fabricius, 1788, declared nomen oblitum. - Ostrea flexuosa Poli, 1795 (currently Flexopecten flexuosus (Poli, 1795)) is declared nomen protectum against the senior synonym Ostrea coarctata Born, 1778, declared nomen oblitum. - Chama aculeata Poli, 1795 (currently Centrocardita aculeata (Poli, 1795)) is declared nomen protectum against the senior homonym Chama aculeata Ström, 1768, declared nomen oblitum, thereby making valid the current usage and avoiding the need for using the junior synonym Centrocardita elegans (Requien, 1848). - Solen marginatus Pulteney, 1799 is declared nomen protectum against the senior syn- onyms Hypogaea tentaculata Poli, 1791, Solen rotundatus Spengler, 1794 and Solen gla- dius Röding, 1798, all declared nomina oblita. -

In Two Different Clam's Beds in Lake Timsah, Suez Canal
Thalassia Salentina Thalassia Sal. 40 (2018), 67-94 ISSN 0563-3745, e-ISSN 1591-0725 DOI 10.1285/i15910725v40p67 http: siba-ese.unisalento.it - © 2018 Università del Salento KANDEEL EL-SAYED KANDEEL Department of Zoology, Faculty of Science, Fayoum University, 63514 Fayoum, Egypt e-mail: [email protected] POPULATION DYNAMICS OF VENERUPIS AUREA (BIVALVIA: VENERIDAE) IN TWO DIFFERENT CLAM’S BEDS IN LAKE TIMSAH, SUEZ CANAL, EGYPT SUMMARY The clam Venerupis aurea (Gmelin, 1971) is one of the most commercially important bivalves in Lake Timsah, Suez Canal. Population dynamics for this clam was explored from August 2015 to September 2016 at two clam beds; Taawen site and Etap site of different sediment characters, population densities and fishing exploitation in the lake. The average density of the species at the exploited Taawen site (3,930 ind. m-2) was significantly high- er (one way ANOVA, P = 5.15) than at unexploited Etap site (1,141 ind. m-2). Length frequency data were analyzed by FiSAT software for estimation of population parameters to evaluate the status of the stock. Asymptotic length (L∞) was similar (36.57 mm) in the two beds. Growth coefficient (K) was higher in Etap site (0.36 yr -1) than in Taawen site (0.28 yr -1). The theoretical lifespan (Tmax) was higher in Taawen site (12.4 years) than in Etap site (9.7 years). Total mortality (Z) was estimated by length-converted catch curve at 0.81 and 0.98 yr -1, fishing mortality (F) at 0.11 and 0.22 yr -1 and natural mortality (M) at 0.70 and 0.76 yr -1 for Taawen site and Etap site, respectively. -

Supplement – December 2017 – Survey of the Literature on Recent
A Malacological Journal ISSN 1565-1916 No. 36 - SUPPLEMENT DECEMBER 2017 2 SURVEY OF THE LITERATURE ON RECENT SHELLS FROM THE RED SEA (third enlarged and revised edition) L.J. van Gemert* Summary This literature survey lists approximately 3,050 references. Shells are being considered here as the shell bearing molluscs of the Gastropoda, Bivalvia and Scaphopoda. The area does not only comprise the Red Sea, but also the Gulf of Aden, Somalia and the Suez Canal, including the Lessepsian species in the Mediterranean Sea. Literature on fossils shells, particularly those from the Holocene, Pleistocene and Pliocene, is listed too. Introduction My interest in recent shells from the Red Sea dates from about 1996. Since then, I have been, now and then, trying to obtain information on this subject. Some years ago I decide to stop gathering data in a haphazard way and to do it more properly. This resulted in a first survey of approximately 1,420 and a second one of 2,025 references (van Gemert, 2010 & 2011). Since then, this survey has again been enlarged and revised and a number of errors have been corrected. It contains now approximately 3,050 references. Scope In principle every publication in which molluscs are reported to live or have lived in the Red Sea should be listed in the survey. This means that besides primary literature, i.e. articles in which researchers are reporting their finds for the first time, secondary and tertiary literature, i.e. reviews, monographs, books, etc are to be included too. These publications were written not only by a wide range of authors ranging from amateur shell collectors to professional malacologists but also people interested in the field of archaeology, geology, etc. -

The Manila Clam Ruditapes Philippinarum (Adams & Reeve, 1850) in the Tagus Estuary (Portugal)
Aquatic Invasions (2017) Volume 12, Issue 2: 133–146 Open Access DOI: https://doi.org/10.3391/ai.2017.12.2.02 © 2017 The Author(s). Journal compilation © 2017 REABIC Research Article Age and growth of a highly successful invasive species: the Manila clam Ruditapes philippinarum (Adams & Reeve, 1850) in the Tagus Estuary (Portugal) Paula Moura1, Lucía L. Garaulet2, Paulo Vasconcelos1,3, Paula Chainho2, José Lino Costa2,4 and Miguel B. Gaspar1,5,* 1Instituto Português do Mar e da Atmosfera (IPMA, I.P.), Avenida 5 de Outubro s/n, 8700-305 Olhão, Portugal 2MARE – Centro de Ciências do Mar e do Ambiente, Faculdade de Ciências, Universidade de Lisboa, Campo Grande, 1749-016 Lisboa, Portugal 3Centro de Estudos do Ambiente e do Mar (CESAM), Departamento de Biologia, Universidade de Aveiro, Campus de Santiago, 3810-193 Aveiro, Portugal 4Departamento de Biologia Animal, Faculdade de Ciências da Universidade de Lisboa, Campo Grande, 1749-016 Lisboa, Portugal 5Centro de Ciências do Mar (CCMAR), Universidade do Algarve, Campus de Gambelas, 8005-139 Faro, Portugal *Corresponding author E-mail: [email protected] Received: 3 November 2016 / Accepted: 4 May 2017 / Published online: 23 May 2017 Handling editor: Philippe Goulletquer Abstract The Manila clam Ruditapes philippinarum (Adams & Reeve, 1850) was introduced in several regions worldwide where it is permanently established. In Portuguese waters, the colonisation of the Tagus Estuary by this invasive species coincided with a significant decrease in abundance of the native Ruditapes decussatus (Linnaeus, 1758). This study aimed to estimate the age and growth of the Manila clam, to compare the growth performance between R.