Some Tanzanite Imitations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
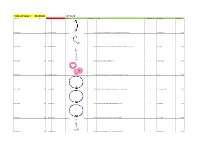
Total Lot Value = $8,189.80 LOT #128 Location Id Lot # Item Id Sku Image Store Price Model Store Quantity Classification Total Value
Total Lot Value = $8,189.80 LOT #128 location_id Lot # item_id sku Image store_price model store_quantity classification Total Value A07-S05-D001 128 182363 TICB-1001 $13.95 16G 5/16" Internally Threaded Implant Grade Titanium Curved Barbell 8 Curved Barbell $111.60 A07-S05-D003 128 186942 NR-1316 $8.95 Purple 2mm Bezel-Set Synthetic Opal Rose Gold-Tone Steel Nose Ring Twist 1 Nose Ring $8.95 A07-S05-D005 128 65492 A208 $11.99 Celtic Swirl Reverse Belly Button Ring 4 Belly Ring Sale $47.96 A07-S05-D006 128 104247 PLG-1345-14 $10.95 9/16" Pink Heart-Shaped Cut Out Flexible Silicone Tunnel Plugs Pair 4 Plugs $43.80 A07-S05-D007 128 65494 D103 $5.95 16G 3/8 - Anodized Rainbow Titanium-Plated Captive Bead Ring 3 Captive Ring , Daith $17.85 A07-S05-D008 128 65496 D103b $5.95 Rainbow Titanium-Plated Captive Bead Ring - 14G 1/2 1 Captive Ring $5.95 A07-S05-D010 128 65499 D103d $4.99 16G 5/8 - Rainbow Anodized Steel Captive Bead Ring 51 Captive Ring $254.49 A07-S05-D011 128 87697 BR-1429 $11.99 Pink CZ Crown & Flower Dangle Belly Button Navel Ring 2 Belly Ring Sale $23.98 A07-S05-D012 128 64066 BR-1496 $11.99 Purple CZ Crown & Flower Dangle Belly Button Navel Ring 4 Belly Ring Sale $47.96 A07-S05-D013 128 65502 D111 $5.99 Spiky -Ball Twister - Body Jewelry 14 Gauge 43 Twister Barbell Sale $257.57 A07-S05-D014 128 104271 PLG-1345-16 $10.95 5/8" - Pink Heart-Shaped Cut Out Flexible Silicone Tunnel Plugs - Pair 2 Plugs $21.90 A07-S05-D015 128 104185 PLG-1345-4 $8.95 6G - Pink Heart-Shaped Cut Out Flexible Silicone Tunnel Plugs - Pair 2 Plugs $17.90 -

Some Uncommon Sapphire “Imitations”: Blue Co-Zirconia, Kyanite & Blue Dumortierite Dr Michael S
Some Uncommon Sapphire “Imitations”: Blue Co-zirconia, Kyanite & Blue Dumortierite Dr Michael S. Krzemnicki Swiss Gemmological Institute SSEF [email protected] 筆者滙報數個瑞士珠寶研究院(SSEF)近期收到 in the ring showed a negative RI reading 要求鑑證的藍色寶石,經檢測後確定其中包括 (above 1.79), an isotropic optical character 一些非常罕見的藍寶石模擬石:含錮氧化鋯、 (polariscope) and thus no pleochroism at all. 藍晶石及藍線石等。 Under the microscope, we saw no inclusions, however a slightly greenish reaction under Sapphires are among the most abundant gems the LWSW and there was a weaker similar we receive at the Swiss Gemmological Institute reaction under SWUV lamps. Based on these (SSEF) for testing. From time to time, however, properties and a chemical analysis by X-ray we are quite surprised by the imitations which fluorescence (EDXRF), the blue stone was we find among the goods sent in and this can readily identified as cubic zirconia (ZrO2). then be disappointing news for the clients. In Having seen this artificial product in a wide the following short note, the author presents a range of colours, the author had not previously few uncommon imitations identified recently at seen one of such a saturated and attractive the SSEF. Identification of these imitations is blue. Based on literature (Nassau 1981) the straightforward and should be no problem for analysed traces of cobalt in that stone have any experienced gemmologist. been identified as the colouring element in this specimen. The absorption spectrum of the stone The first case is that of an attractive blue (Fig. 2) – although superposed by several rare faceted stone of approximately 1.4 ct, set in a ring with diamonds (Fig. -

Compilation of Reported Sapphire Occurrences in Montana
Report of Investigation 23 Compilation of Reported Sapphire Occurrences in Montana Richard B. Berg 2015 Cover photo by Richard Berg. Sapphires (very pale green and colorless) concentrated by panning. The small red grains are garnets, commonly found with sapphires in western Montana, and the black sand is mainly magnetite. Compilation of Reported Sapphire Occurrences, RI 23 Compilation of Reported Sapphire Occurrences in Montana Richard B. Berg Montana Bureau of Mines and Geology MBMG Report of Investigation 23 2015 i Compilation of Reported Sapphire Occurrences, RI 23 TABLE OF CONTENTS Introduction ............................................................................................................................1 Descriptions of Occurrences ..................................................................................................7 Selected Bibliography of Articles on Montana Sapphires ................................................... 75 General Montana ............................................................................................................75 Yogo ................................................................................................................................ 75 Southwestern Montana Alluvial Deposits........................................................................ 76 Specifi cally Rock Creek sapphire district ........................................................................ 76 Specifi cally Dry Cottonwood Creek deposit and the Butte area .................................... -

HK Fancy Sapphire
Hong Kong, March 2011 Fancy Coloured Sapphires: The Beauty beyond "Blue" of Sapphire and "Red" of Ruby Dr. Michael S. Krzemnicki Swiss Gemmological Institute SSEF Switzerland All photos © M.S. Krzemnicki, SSEF except where indicated. The range of colours... © Swiss Gemmological Institute SSEF 1 The range of colours... © Swiss Gemmological Institute SSEF The range of colours... © Swiss Gemmological Institute SSEF 2 The range of colours... © Swiss Gemmological Institute SSEF The range of colours... © Swiss Gemmological Institute SSEF 3 The range of colours... © Swiss Gemmological Institute SSEF The range of colours... © Swiss Gemmological Institute SSEF 4 The range of colours... © Swiss Gemmological Institute SSEF The range of colours... © Swiss Gemmological Institute SSEF 5 The range of colours... © Swiss Gemmological Institute SSEF The range of colours... © Swiss Gemmological Institute SSEF 6 The range of colours... © Swiss Gemmological Institute SSEF The range of colours... © Swiss Gemmological Institute SSEF 7 The range of colours... © Swiss Gemmological Institute SSEF The range of colours... © Swiss Gemmological Institute SSEF 8 The range of colours... © Swiss Gemmological Institute SSEF The range of colours... Fancy sapphires: The colour range beyond red of rubies and blue of sapphires © Swiss Gemmological Institute SSEF 9 The range of colours... Photo: © SilkenEast Ltd, Bangkok © Swiss Gemmological Institute SSEF The range of colours... Collection: SilkenEast Ltd, Bangkok © Swiss Gemmological Institute SSEF 10 Jewellery with fancy sapphires Photos © Luc Phan, SSEF © Swiss Gemmological Institute SSEF © Swiss Gemmological PhotoInstitute © Luc SSEF Phan, SSEF 11 Corundum Chemical composition: aluminium oxide, Al2O3 Chemical pure aluminium oxide is colourless © Wikipedia In nature always with trace elements (chemical impurities), usually: - Mg, Ti, V, Cr, Fe, Ga - and occasionally rare HFS-elements such as Nb, Sn, Ta, Th Not all trace elements are affecting the colour (e.g. -

Metamorphic and Metasomatic Kyanite-Bearing Mineral
Metamorphic and Metasomatic Kyanite-Bearing Mineral Assemblages of Thassos Island (Rhodope, Greece) Alexandre Tarantola, Panagiotis Voudouris, Aurélien Eglinger, Christophe Scheffer, Kimberly Trebus, Marie Bitte, Benjamin Rondeau, Constantinos Mavrogonatos, Ian Graham, Marius Etienne, et al. To cite this version: Alexandre Tarantola, Panagiotis Voudouris, Aurélien Eglinger, Christophe Scheffer, Kimberly Tre- bus, et al.. Metamorphic and Metasomatic Kyanite-Bearing Mineral Assemblages of Thassos Island (Rhodope, Greece). Minerals, MDPI, 2019, 10.3390/min9040252. hal-02932247 HAL Id: hal-02932247 https://hal.archives-ouvertes.fr/hal-02932247 Submitted on 7 Sep 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. minerals Article Metamorphic and Metasomatic Kyanite-Bearing Mineral Assemblages of Thassos Island (Rhodope, Greece) Alexandre Tarantola 1,* , Panagiotis Voudouris 2 , Aurélien Eglinger 1, Christophe Scheffer 1,3, Kimberly Trebus 1, Marie Bitte 1, Benjamin Rondeau 4 , Constantinos Mavrogonatos 2 , Ian Graham 5, Marius Etienne 1 and Chantal Peiffert -

Distinctive Designs
Distinctive Designs Brides Ring in an Era of Self-Expression by Stacey Marcus Diamond rings have been sporting the hands of newly can elevate a traditional setting to an entirely new level. While engaged women since ancient times. In the mid 1940s, diamonds are always in vogue for engagement rings, millen- DeBeers revived the ritual with its famous “Diamonds Are nials are also opting for nontraditional stones, such as color- Forever” campaign. Today’s brides are blazing new trails by changing alexandrite, beautiful tanzanite, black opal, and even selecting nontraditional wedding rings that express their aquamarine. We are in an age when anything goes, and brides individual style. are embracing the idea of individuality and self-expression!” True Colors says Jordan Fine, CEO of JFINE. “Brides today want to be unique and they don’t mind taking Kathryn Money, vice president of strategy and merchandising chances with colors, settings, and stones. When it comes to at Brilliant Earth agrees: “Customers are seeking products that choosing an engagement ring, natural pink, blue, and even express their individuality and are increasingly drawn towards green diamonds are trending. These precious and rare dia- uniqueness. They want a ring that isn’t like everyone else’s, monds originate from only a few mines in the world, and they which we’re seeing manifested in many different ways, such 34 Spring 2018 BRIDE&GROOM as choosing a distinctive ring setting, using colored gemstones in lieu of a diamond, using a fancy (non-round) diamond, or opting for rose gold.” Money adds that 16% of respondents in a recent survey they conducted favored a colored gemstone engagement ring over a diamond. -

Scientific Communication
SCIENTIFIC COMMUNICATION NOTES ON FLUID INCLUSIONS OF VANADIFEROUS ZOISITE (TANZANITE) AND GREEN GROSSULAR IN MERELANI AREA, NORTHERN TANZANIA ELIAS MALISA; KARI KINNUNEN and TAPIO KOLJONEN Elias Malisa: University of Helsinki, Department of Geology, SF-00170 Helsinki, Finland. Kari Kinnunen and Tapio Koljonen: Geological Survey of Finland, SF-02150 Espoo, Finland. Tanzanite is a trade name for a gem-quality has been reported in Lalatema and Morogoro in vanadiferous zoisite of deep sapphire-blue colour Tanzania and in Lualenyi and Lilani in Kenya discovered in Merelani area, Tanzania in 1967. (Naeser and Saul 1974; Dolenc 1976; Pohl and This mineral was first described as a strontium Niedermayr 1978). -bearing zoisite by Bank, H. & Berdesinski, W., Crystals of tanzanite occur mainly in bou- 1967. Other minor occurrences of this mineral dinaged pegmatitic veins and hydrothermal frac- Fig. 1. Tanzanite-bearing horizon in the graphite-rich diopside gneiss. The yellow colour indicates hydrothermal alteration, which can be used in pros- pecting for tanzanite. Length of photo ca. 8 m. 54 Elias Malisa, Kari Kinnunen and Tapio Koljonen given as Ca2Al3Si30120H (Ghose & Tsang 1971). The chemical compositions of tanzanites studied are given in Table 1. Unit cell dimensions, measured by X-ray dif- fraction, are a = 16.21, b = 5.55, c = 10.03 ± 0.01 Å in agreement with Hurlbut (1969). Zoisite shows diffraction symmetry mmmPn-a, which limits the possible space groups to Pnma if centric or Pn2, if acentric (Dallace 1968). The most striking property of tanzanite is its pleochroism, which changes from trichroic to dichroic on heating; normally its pleochroism varies: X = red-violet, Y = c = deep blue, Z = a = yellow- Fig. -

MAGAZINE • LEAWOOD, KS SPECIAL EDITION 2016 ISSUE 3 Fine Jewelers Magazine
A TUFTS COMMUNICATIONS fine jewelry PUBLICATION MAZZARESE MAGAZINE • LEAWOOD, KS SPECIAL EDITION 2016 ISSUE 3 fine jewelers magazine Chopard: Racing Special The New Ferrari 488 GTB The Natural Flair of John Hardy Black Beauties Omega’s New 007 SPECIAL EDITION 2016 • ISSUE 3 MAZZARESE FINE JEWELERS MAGAZINE • SPECIAL EDITION 2016 welcome It is our belief that we have an indelible link with the past and a responsibility to the future. In representing the fourth generation of master jewelers and craftsmen, and even as the keepers of a second generation family business, we believe that the responsibility to continually evolve and develop lies with us. We endeavor to always stay ahead of the latest jewelry and watch trends and innovations. We stay true to our high standards and objectives set forth by those that came before us by delivering a jewelry experience like no other; through the utmost attention to service, knowledge and value. Every great story begins with a spark of inspiration. We are reminded day after day of our spark of inspiration: you, our esteemed customer. Your stories help drive our passion to pursue the finest quality pieces. It is a privilege that we are trusted to provide the perfect gift; one that stands the test of time and is passed along through generations. We find great joy in assisting the eager couple searching for the perfect engagement ring, as they embark on a lifetime of love, helping to select a quality timepiece, or sourcing a rare jewel to mark and celebrate a milestone. We are dedicated to creating an experience that nurtures relationships and allows those who visit our store to enter as customers, but leave as members of our family. -

VOLUME 45, NO. 80 PLEOCHRONIC MINERALS Wednesday July 28 7:00—9:00 Pm Makiki District Park Administration Building NEXT MONTH
VOLUME 45, NO. 80 JULY 2010 PLEOCHRONIC MINERALS MEETING BY DEAN SAKABE Wednesday Pleochroic minerals are miner- July 28 als that show different colors 7:00—9:00 pm depending on what direction Makiki District you are observing the crystal. Park In order to view pleochroism Administration you need an individual transpar- Building ent crystal. This effect can be very dramatic. Many minerals NEXT MONTH are technically pleochroic, but Wednesday most often the color change is August 25, 2010 so small that it can be barely detected. For those few other LAPIDARY minerals, the color change is very, very obvious. The great- Every Thursday est change is limited to three 6:30-8:30pm colors and is called trichroic(1-3). Second-floor Arts A two color change occurrence and Crafts Bldg is called dichroic (4-5). Pleo- Makiki District chroic, which means "many col- Park ors", is used to cover both of 1-3 Tanzanite with all 3 colors of the natural these color changes. Most of trichroic crystal present and strongly show- ing down different axes of view the time, the color change is MEMBERSHIP limited to shade changes such COSTS as from pale pink to dark pink. 2008 Single: $10.00 Family: $15.00 Rock and Mineral Society of Hawai‛i INC. PLEOCHRONIC MINERALS , PAGE 2 rhombic, monoclinic, and triclinic minerals that can be trichroic. This is because they have three unique axes of symmetry and therefore three unique directions that can absorb light in three different ways. The most famous dichroic mineral is Cordierite, a Magne- sium Aluminum Silicate. -

Evaluation of Brilliance, Fire, and Scintillation in Round Brilliant
Optical Engineering 46͑9͒, 093604 ͑September 2007͒ Evaluation of brilliance, fire, and scintillation in round brilliant gemstones Jose Sasian, FELLOW SPIE Abstract. We discuss several illumination effects in gemstones and University of Arizona present maps to evaluate them. The matrices and tilt views of these College of Optical Sciences maps permit one to find the stones that perform best in terms of illumi- 1630 East University Boulevard nation properties. By using the concepts of the main cutter’s line, and the Tucson, Arizona 85721 anti-cutter’s line, the problem of finding the best stones is reduced by E-mail: [email protected] one dimension in the cutter’s space. For the first time it is clearly shown why the Tolkowsky cut, and other cuts adjacent to it along the main cutter’s line, is one of the best round brilliant cuts. The maps we intro- Jason Quick duce are a valuable educational tool, provide a basis for gemstone grad- Jacob Sheffield ing, and are useful in the jewelry industry to assess gemstone American Gem Society Laboratories performance. © 2007 Society of Photo-Optical Instrumentation Engineers. 8917 West Sahara Avenue ͓DOI: 10.1117/1.2769018͔ Las Vegas, Nevada 89117 Subject terms: gemstone evaluation; gemstone grading; gemstone brilliance; gemstone fire; gemstone scintillation; gemstone cuts; round brilliant; gemstones; diamond cuts; diamonds. James Caudill American Gem Society Advanced Instruments Paper 060668R received Aug. 28, 2006; revised manuscript received Feb. 16, 8881 West Sahara Avenue 2007; accepted for publication Apr. 10, 2007; published online Oct. 1, 2007. Las Vegas, Nevada 89117 Peter Yantzer American Gem Society Laboratories 8917 West Sahara Avenue Las Vegas, Nevada 89117 1 Introduction are refracted out of the stone. -

Murostar-Katalog-2018-2019.Pdf
Material B2B Großhandel für Piercing- und Tattoo- B2B Wholesale for body piercing and Unsere Qualität ist Ihre Zufriedenheit Our quality is your satisfaction About Us studios, sowie Schmuck- und Juwelier- tattoo studios as well as jewelry stores vertriebe Oberste Priorität hat bei uns die reibungslose Abwicklung Our top priority is to provide a smooth transaction and fast Titan Produkte entsprechen dem Grad 23 (Ti6AL 4V Eli) und Titanium products correspond to titanium grade 23 (Ti6Al und schnelle Lieferung Ihrer Ware. Daher verlassen ca. 95 % delivery. Therefore, approximately 95% of all orders are sind generell hochglanzpoliert. Sterilisierbar. 4V Eli) and are generally high polished. For sterilization. aller Aufträge unser Lager noch am selben Tag. shipped out on the same day. Black Titan besteht grundsätzlich aus Titan Grad 23 (Ti6AL Black Titanium is composed of titanium grade 23 (Ti6Al 4V Unser dynamisches, kundenorientiertes Team sowie ausge- Our dynamic, customer-oriented team and excellent expe- 4V Eli) und ist zusätzlich mit einer PVD Titanium Beschich- Eli) and is additionally equipped with a PVD Black Titanium zeichnete Erfahrungswerte garantieren Ihnen einen hervor- rience guarantee excellent service and expert advice. tung geschwärzt. Sterilisierbar. Coating. For sterilization. ragenden Service und kompetente Beratung. Steel Schmuck besteht grundsätzlich aus Chirurgenstahl Steel jewelry is composed of 316L Surgical Steel and is also Sie bestellen schnell und unkompliziert online, telefo- Your order can be placed quickly and easily online, by 316L und ist ebenfalls hochglanzpoliert. Sterilisierbar. high polished. For sterilization. nisch, per Fax oder Email mittels unseres einfachen Ex- phone, fax or email using our simple Express Number press-Nummern-Systems ohne Angabe von Farb- oder Grö- System only without providing colors or sizes. -

Across the Cascade Range
Series I B> DescriPtive Geology- 4l Bulletin No. 235 \ D, Petrography and Mineralogy, DEPARTMENT'OF THE INTERIOR UNITED STATES GEOLOGICAL SURVEY CHARLES \). WALCOTT, Di HECTOR GEOLOGICAL RECONNAISSANCE ACROSS THE CASCADE RANGE NEAR THE FORTY-NINTH PARALLEL GEORGE OTIS SMITH AND FRANK C. CALKINS WASHINGTON GOVERNMENT PRINTING OFFICE 1904 Trri-o^) SL'BD C 0 N T E N T S. I'lliJO. Letter of transmittal. ---_--_---..-.._-_.____.._-______._....._.._____.._.. 9 Introduction-__-._.__,.__-.----._--._._.__..._....__....---_--__._.__.-.-_- 11 Scope of report ---.--_.____.._______-.--....._---.._...._.__ ._.- 11 Route followed ........................:......................... 12 Geography .............................................................. 12 Topography .......................................................... 12 Primary divisions of the region..--.........-.--.-.--.-.-.. 12 Okanogan Valley .................:.. ............................ 18 Cascade Range ...............:........,..._ ....^......i........ 13 General characteristics..._.....-.....-..----.--.----.-.-..-.. 13 Northern termination.,.---.....-......--.-.............._ 13 Subdivision .............................................. 14 Okanogan Mountains ........................................... 14 Hozonieen Range ............................................ 15 Skagit Mountains....-.... ......-.----....-.-----..-...--.--- 16 Drainage ..................................................... 17 Climate ...................................................... ...... 17 Roads and trails