Quartz: a Bull's Eye on Optical Activity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Compilation of Reported Sapphire Occurrences in Montana
Report of Investigation 23 Compilation of Reported Sapphire Occurrences in Montana Richard B. Berg 2015 Cover photo by Richard Berg. Sapphires (very pale green and colorless) concentrated by panning. The small red grains are garnets, commonly found with sapphires in western Montana, and the black sand is mainly magnetite. Compilation of Reported Sapphire Occurrences, RI 23 Compilation of Reported Sapphire Occurrences in Montana Richard B. Berg Montana Bureau of Mines and Geology MBMG Report of Investigation 23 2015 i Compilation of Reported Sapphire Occurrences, RI 23 TABLE OF CONTENTS Introduction ............................................................................................................................1 Descriptions of Occurrences ..................................................................................................7 Selected Bibliography of Articles on Montana Sapphires ................................................... 75 General Montana ............................................................................................................75 Yogo ................................................................................................................................ 75 Southwestern Montana Alluvial Deposits........................................................................ 76 Specifi cally Rock Creek sapphire district ........................................................................ 76 Specifi cally Dry Cottonwood Creek deposit and the Butte area .................................... -

Evaluation of Brilliance, Fire, and Scintillation in Round Brilliant
Optical Engineering 46͑9͒, 093604 ͑September 2007͒ Evaluation of brilliance, fire, and scintillation in round brilliant gemstones Jose Sasian, FELLOW SPIE Abstract. We discuss several illumination effects in gemstones and University of Arizona present maps to evaluate them. The matrices and tilt views of these College of Optical Sciences maps permit one to find the stones that perform best in terms of illumi- 1630 East University Boulevard nation properties. By using the concepts of the main cutter’s line, and the Tucson, Arizona 85721 anti-cutter’s line, the problem of finding the best stones is reduced by E-mail: [email protected] one dimension in the cutter’s space. For the first time it is clearly shown why the Tolkowsky cut, and other cuts adjacent to it along the main cutter’s line, is one of the best round brilliant cuts. The maps we intro- Jason Quick duce are a valuable educational tool, provide a basis for gemstone grad- Jacob Sheffield ing, and are useful in the jewelry industry to assess gemstone American Gem Society Laboratories performance. © 2007 Society of Photo-Optical Instrumentation Engineers. 8917 West Sahara Avenue ͓DOI: 10.1117/1.2769018͔ Las Vegas, Nevada 89117 Subject terms: gemstone evaluation; gemstone grading; gemstone brilliance; gemstone fire; gemstone scintillation; gemstone cuts; round brilliant; gemstones; diamond cuts; diamonds. James Caudill American Gem Society Advanced Instruments Paper 060668R received Aug. 28, 2006; revised manuscript received Feb. 16, 8881 West Sahara Avenue 2007; accepted for publication Apr. 10, 2007; published online Oct. 1, 2007. Las Vegas, Nevada 89117 Peter Yantzer American Gem Society Laboratories 8917 West Sahara Avenue Las Vegas, Nevada 89117 1 Introduction are refracted out of the stone. -

General Information- Michigan's Gem Stones
CONTENTS: INDUSTRIES MAP —————— FACING I GENERAL INFORMATION- —————— I MICHIGAN'S GEM STONES- —————— 4 ASSAYS AND TESTS———— ————— I I ARTICLES, PERIODICALS, BOOKS- —————12 SOCIETIES—————————————————— ———— 23 MUSEUMS—————————————————— ———— 26 GEOLOGIC TIME SCALE ———— 30 GEOLOGIC MAP —————— ————— 3 I NMICHIGAN DEPARTMENT OF CONSERVATION ^GEOLOGICAL SURVEY DIVISION FREE DISTRIBUTION ONLY PREFACE TO THIRD EDITION The first edition, published in April, 1958 was needed in responding to queries following a mineral show on the Depart- ment T-V program "Michigan Conservation11. The second edition, July, 1959) was characterized by the addition of the section on gem stones* An abstracted version of the second edition titled "Pebbles to Pendants" was published in the July, 1958 issue of "Michigan Conservation". The present 'third edition is another major revision. Among the new materials added are: l) bedrock geologic map, 2) mineral industries map, 3) rock column and time scale, 4) mineral and fossil sketches, and 5) locality sketch maps* These, along with the cover, were prepared by Jim Campbell of our staff. The book list has been expanded and several titles were added to the articles list. Suggestions received from Arthur Johnstone, of the Michigan Mineralogical Society, were particularly helpful. Information regarding mineral and lapidary businesses may be found in the appropriate advertising media as well as from many of the clubs. Robert W. Kelley, Geologist Geological Survey Division Michigan Dept. Conservation March, I960 Lansing MICHIGAN'S MIN ERAL EXTRACTING INDUSTRIES D NON-METALLIC B BRINE H SALT 0 CLAY 0 SHALE 9 DOLOMITE H LIMESTONE 13 SAND& GRAVELCPRINCIPAL AREA) H GLASS SAND 0 GYPSUM B SANDSTONE D MARLCPRINCIPAL AREA) B PEAT • MISCELLANEOUS STONE A METALLIC L COPPER A IRON O FUELS <D GAS • OIL MAJOR FIELDS € OIL & GAS INDIANA O H I O GENERAL INFORMATION INTRODUCTION Interest in collecting minerals and gem stones and in doing lapidary work certainly is on the increase today. -

Optical Mineralogy in a Nutshell
Optical Mineralogy in a Nutshell Use of the petrographic microscope Slides borrowed/adapted from Jane Selverstone (University of New Mexico) and John Winter (Whitman College) Why use the petrographic microscope? • Identify minerals (no guessing!) • Determine rock type • Determine crystallization sequence • Document deformation history • Observe frozen-in reactions • Constrain P-T history • Note weathering/alteration • Fun, powerful, and cheap! The petrographic microscope Also called a polarizing microscope In order to use the scope, we need to understand a little about the physics of light, and then learn some tools and tricks… Polarized Light Microscopy Isotropic materials, which include gases, liquids, unstressed glasses and cubic crystals, demonstrate the same From Nikon optical properties in all directions. They have only one refractive index and no restriction on the vibration direction of light passing through them. Anisotropic materials, in contrast, which include 90 percent of all solid substances, have optical properties that vary with the orientation of incident light with the crystallographic axes. Anisotropic materials act as beam splitters and divide light rays into two parts. The technique of polarizing microscopy exploits the interference of the split light rays, as they are re-united along the same optical path to extract information about these materials. What happens as light moves through the scope? plane polarised light (single vibration direction) unpolarised light (all possible vibration directions) 1) Light passes -

Syllabus Optical Mineralogy
CRYSTALLOGRAPHY UNIT IV UNIT-IV: SYLLABUS OPTICAL MINERALOGY : Light: Corpuscular, electromagnetic and quantum theories. Ordinary light and plane polarized light. Refractive index an its determination: Relief method, Becke line, Central illumination, and Oblique illumination methods. Isotropism, isotropic minerals and isotropic ray velocity surface. Behaviour of light in isotropic minerals. Petrological Microscope and its parts-optical accessories and their uses:Quartz wedge, Gypsum plate and Mica plate. Study of Isotropic minerals using the petrological microscope: properties of isotropic minerals under parallel Nicol conditions. LightCorpuscular A source of light continuously emits tiny elastic particles called corpuscles. These particles or the corpuscles moves with high velocity as that of light and gets scattered in all directions of light. This theory says that the velocity of light changes with the change in density of the medium in which it is used. This theory could explain three main phenomena of light that is reflection, refraction, and rectilinear propagation of light. This theory also says that the color of light is dependent on the size of the corpuscles. Some of the main drawbacks of this theory are 1) This theory could not explain the phenomena of interference, diffraction, and polarization of light etc. 2) According to this theory the velocity of light in denser medium is greater than the velocity of light in rarer medium but this is proved wrong later 3) This theory assumes that the source of light looses the mass as it emits corpuscles; but not such determent in mass of the source of light is detected. 4) This theory proposes that velocity of the corpuscles increases as the temperature of the source increases as the temperature increases experiments have proved that the velocity of light is independent of temperature. -

GEOL 221 Mineralogy and Mineral Optics Course Syllabus
SAN DIEGO STATE UNIVERSITY GEOL 221 Mineralogy and Mineral Optics Course Syllabus Instructor: Professor David L. Kimbrough Email: [email protected], Phone: 594-1385 Lecture: MW 1000-1050 CSL 422, Lab: W 1400-1640, Lab study: M 1400-1640 CSL 425 Office: GMCS-229A; Office hours: MW 1100-1200; T 1115-1215; by appointment TA: Mark Nahabidian GMCS-133 TBA Course Prerequisite: Chem 200 or concurrent registration. Credit or concurrent registration in OCEAN 100 or GEOL 100 and 101 or GEOL 104 and 101; Geol 200; Required Texts: Introduction to Mineralogy, William D. Nesse, Oxford University Press. Minerals in Thin Section, Perkins & Henke, Prentice Hall Recommended Text: Dictionary of Geological Terms, AGI Bates & Jackson eds. or similar, The Complete Guide to Rocks & Minerals by John Farndon or similar Other required materials: Hand lens; calculator Classroom management: Attendance is crucial. Please let me know if you’re going to be absent for any reason. Be on time for class, don’t participate in excessive side-chatter or cause disruptions during class. Always respond to the instructor, the Teaching Assistant, and your fellow students in a respectful and civil manner. Cheating or plagiarism is not tolerated. It’s easy to spot and constitutes serious academic misconduct. Helpful hints to make Mineralogy easier and more fun! Attend class: Studies show that the most valuable time commitment by students in a course is the time actually spent in the classroom. Class time is the most important determinant of student success and yields the greatest improvement in student learning outcomes. Information is covered in class that is not in the textbook, and parts of the book are hard to understand. -
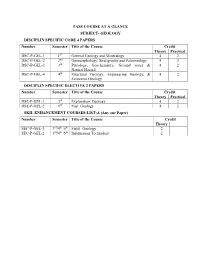
Pass Course at a Glance
PASS COURSE AT A GLANCE SUBJECT- GEOLOGY DISCIPLIN SPECIFIC CORE 4 PAPERS Number Semester Title of the Course Credit Theory Practical DSC-P-GEL-1 1ST General Geology and Mineralogy 4 2 DSC-P-GEL-2 2nd Geomorphology, Stratigraphy and Paleontology 4 2 DSC-P-GEL-3 3rd Petrology, Geochemistry, Ground water & 4 2 Natural Hazard DSC-P-GEL-4 4th Structural Geology, Engineering Geology, & 4 2 Economic Geology DISCIPLIN SPECIFIC ELECTIVE 2 PAPERS Number Semester Title of the Course Credit Theory Practical DSE-P-GEL-1 5th Exploration Geology 4 2 DSE-P-GEL-2 6th Fuel Geology 4 2 SKIL ENHANCEMENT COURSES-LIST-A (Any one Paper) Number Semester Title of the Course Credit Theory SEC-P-GEL-1 3rd/4th /5th Field Geology 2 SEC-P-GEL-2 3rd/4th /5th Information Technology 2 FIRST SEMESTER GEOLOGY PASS PAPER-I Theory Paper-I (General Geology, Crystallography and Mineralogy) Objectives of the Course: The aim of this course is to study General geology part can give an idea about endogenetic process operating inside the earth. study the crystals through external elements of symmetry, crystal classes and systems, and the relations of symmetry to the internal structure using the chemical and physical properties of the minerals. The course aims also to study the major mineral groups, their occurrences, physical, chemical and crystallographic properties and their possible uses in industry. In these units, the physical, chemical and optical properties of the minerals are described. One should know them to identify the types of rocks. Expected outcome: The said courses will make the students to understand about the interior of earth. -

Gemology with Kelly Sitek Ologies Podcast October 10, 2017
Gemology with Kelly Sitek Ologies Podcast October 10, 2017 Heeey, okay just a quick note up top, that this episode gets a little, like, woo-doo-to-doo, like a little mystical. We don’t not talk about the power of crystals in this. I wanted to know, as a gemologist, what this Ologist’s belief was in the mystical nature of rocks, if she believed in it. So, I hear her out, I also discuss the neuroscience of the placebo effect and how our thoughts can change our behaviors. It’s based in neuroscience. Try not to @ me about it ‘cause, like, I get it. Okay? Cool Episode 4 of Ologies, comin’ in hawt. First off, thanks to everyone who’s been listening and leaving reviews on iTunes, and rating, and subscribing. The more you do that, the higher this gets on the charts and the more people see it. And the more people share dumb science jokes, I guess, the better. I don't know. Also thank you to everyone who’s supporting on Patreon. I see you and I love you. And For everyone who had a hankering for merch and who’s been to OlogiesMerch.com. Cool shirt, or mug, or tote. Okay. Gems. This episode about gems is truly outrageous. Well, it’s pretty good. We don’t talk about crotches very much, but it's a pretty good episode. Let’s start with the etymology of gemology, it comes from ‘ology,’ the study of, and ‘gems,’ meaning gems. But ‘gems’ comes from an old dusty Latin word for.. -

Optical Mineralogy in a Nutshell
Optical Mineralogy in a Nutshell Use of the petrographic microscope in three easy lessons Part III © Jane Selverstone, University of New Mexico, 2003 A few new properties, and then some review… Cleavage – number and orientation of cleavage planes Twinning – type of twinning, orientation Extinction angle – parallel or inclined? Angle? Habit – characteristic form of mineral Cleavage Most easily observed in PPL (upper polarizer out), but visible in XN as well • No cleavages: quartz, olivine • 1 good cleavage: micas • 2 good cleavages: pyroxenes, amphiboles Cleavage 2 cleavages intersecting at ~90° pyroxene 120° 2 cleavages 60° intersecting at 60°/120°: amphibole Cleavage random fractures, no cleavage: olivine Twinning Presence and style of twinning can be diagnostic Twins are usually most obvious in XN (upper polarizer in) Twinning - some examples Clinopyroxene (augite) • Simple twin on {100} Plagioclase • Simple (Carlsbad) twin on (010) • Polysynthetic albite twins on (010) • Pericline twin on (h01) Extinction angle Extinction behavior is a function of the relationship between indicatrix orientation and crystallographic orientation parallel extinction inclined extinction Extinction angle – parallel extinction • All uniaxial minerals show parallel extinction • Orthorhombic minerals show parallel extinction (this is because the crystal axes and indicatrix axes coincide) orthopyroxene PPL XN Extinction angle - inclined extinction Monoclinic and triclinic minerals: indicatrix axes do not coincide with crystallographic axes These minerals have -

Short History of Teaching Mineralogy at the Eötvös Loránd University, Budapest
Acta Mineralogica-Petrographica, Szeged 2004, Vol. 45/1, pp. 5-20 1 SHORT HISTORY OF TEACHING MINERALOGY AT THE EÖTVÖS LORÁND UNIVERSITY, BUDAPEST 1 2 1 GY. BUDA , G. PAPP , T. G. WEISZBURG Megjegyzés: Kérem a teljes neveket! 1 Department of Mineralogy, Eötvös Loránd University, H-1117 Budapest, Pázmány Péter sétány 1/C, Hungary 2 Department of Mineralogy and Petrology, Hungarian Natural History Museum, H-1431Budapest. Pf.:137, Hungary e-mail: [email protected] We intend to overview the 230-year history of organised teaching of mineralogy at the Eötvös Loránd University. The University was founded in 1635. Students could learn certain elements of mineralogy already in the early period of the University within the frame of physics. Mineralogy, as an independent subject, has been part of the curriculum since 1774, the year when the Department of Natural History was founded. The separate Department of Mineralogy was established in 1849. While trying to divide the long historical span into periods, no unique concept appropriate in every respect was found, so changes of institutional structure, as well as the prominent mineralogy-related personalities are used as guidelines. For helping the reader not experienced in historical and cultural development of that part of Central Europe, at some points we are giving also explanatory notes related to political, cultural and science history. EARLY PERIOD OF TEACHING MINERALOGICAL KNOWLEDGE the Jesuit Ratio Studiorum (1599). Mineralogy was not (MINERALOGY AS PART OF PHYSICS, 1635–1774) included directly in the curriculum, but in books of some In 1635, when Péter Pázmány, archbishop of Esztergom, professors (e.g. -

Weight of Production of Emeralds, Rubies, Sapphires, and Tanzanite from 1995 Through 2005
Weight of Production of Emeralds, Rubies, Sapphires, and Tanzanite from 1995 Through 2005 By Thomas R. Yager, W. David Menzie, and Donald W. Olson Open-File Report 2008–1013 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior DIRK KEMPTHORNE, Secretary U.S. Geological Survey Mark D. Myers, Director U.S. Geological Survey, Reston, Virginia: 2008 For product and ordering information: World Wide Web: http://www.usgs.gov/pubprod Telephone: 1-888-ASK-USGS For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment: World Wide Web: http://www.usgs.gov Telephone: 1-888-ASK-USGS Suggested citation: Yager, T.R., Menzie, W.D., and Olson, D. W., 2008, Weight of production of emeralds, rubies, sapphires, and tanzanite from 1995 through 2005: U.S. Geological Survey Open-File Report 2008-1013, 9 p., available only online, http://pubs.usgs.gov/of/2008/1013. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this report is in the public domain, permission must be secured from the individual copyright owners to reproduce any copyrighted material contained within this report. ii Contents Introduction ...................................................................................................................................1 Emeralds.......................................................................................................................................2 -

1 Optical Properties of Gem Substances
Optical Properties of Gem Substances Educational objective: This exercise, unit 7 in the above syllabus, will introduce students to skills and equipment that will continue to be used throughout the following modules. You should attempt to master these skills and the use of the equipment. The student should become familiar with the equipment, its application to solving gem problems, its proper use, and the precautions necessary to ensure both the safety of the student and the equipment. This lab may take up to two full meetings. Depending on equipment availability and time one or two setups for each experiment may be present. For each lab experiment, the student group will consist of 2 students. Groups are requested to make a 5 to 10 minute presentation using the digital Elmo overhead projector or a series of digital images (as in previous labs). There are some guiding questions for each exercise, but your overall grade is based on your presentation and discussion ensuing from it, plus observations of your behavior and preparedness in the lab. Images for your presentation are available in a “lab library” of images that you can pick and choose from as you see fit. The order in which the experiments will be presented does not have to correspond with the order they are presented here. 1. Refraction and the critical angle. Objectives. To observe the change in light’s path as it moves from one substance to another, record the angle of incidence and refraction, see total internal reflection, attempt to measure the critical angle, and learn about refractive index.