Igneous Petrology 2001
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Syllabus of Geology
THE UNIVERSITY OF BURDWAN Syllabus for 3-Year Degree Course in Geology (Honours) Full Marks: 800 Distribution of Papers, Marks And Lectures/Periods PART I EXAMINATION (EXAMINATION AT THE END OF FIRST YEAR) Theory Subject Marks No. of lectures Paper-I A. Crystallogroaphy (10) & Mineralogy (30) 40 80 B. Igneus Petrology- I 20 40 C. Metamorphic Petrology-I 20 40 D. Sedimentology-I 20 40 TOTAL 100 Paper-II A. Earth System Sciences 20 40 B. Structural Geology-I 20 40 C. Principles of Stratigraphy 10 20 TOTAL 50 Practical Paper-I Crystallography 05 Hand specimens of minerals 10 Hand specimens of rocks 15 Identification of minerals under microscope 10 Field Report 05 Laboratory Note Book 05 TOTAL 50 150 GRAND TOTAL 200 PART II EXAMINATION (EXAMINATION AT THE END OF SECOND YEAR) Theory Subject Marks No. of lectures Paper-III A. Igneous Petrology-II 20 40 B. Metamorphic Petrology-II 20 40 C. Geochemistry 30 60 D. Geotectonics 30 60 TOTAL 100 Paper-IV A. Structural Geology-II 20 40 B. Sedimentology-II 20 40 C. Hydrogeology 10 20 TOTAL 50 Practical Paper-II Structural Geology-I 35 Field Report 10 Laboratory Note Book 05 TOTAL 50 150 GRAND TOTAL 200 1 PART III EXAMINATION (EXAMINATION AT THE END OF THIRD YEAR) Theory Subject Marks No. of lectures Paper-V A. Principles of Palaeonotology 20 40 B. Palaeonotology 30 60 C. Indian Stratigraphy 50 100 TOTAL 100 Paper-VI A. Economic Geology 50 100 B. Fuels 20 40 C. Natural Resources Management 30 60 TOTAL 100 Practical Paper-III Petrography of Igneous rocks 15 Petrography of Metamorphic rocks 15 Special optics 15 Laboratory Note Book 05 TOTAL 50 150 Paper-IV Sedimentology 15 Remote Sensing 15 Hydrogeology 15 Laboratory Note Book 05 TOTAL 50 150 Paper-V Structural Geology-II 35 Field Report 10 Laboratory Note Book 05 TOTAL 50 150 Paper-VI Palaeonotology 45 Laboratory Note Book 05 TOTAL 50 150 GRAND TOTAL 400 2 DETAILS OF THE THREE YEAR B.Sc. -

Introduction to Igneous Petrology
Cambridge University Press 978-1-107-02754-1 - Essentials of Igneous and Metamorphic Petrology B. Ronald Frost and Carol D. Frost Excerpt More information Chapter 1 Introduction to Igneous Petrology 1.1 Introduction Igneous petrology is the study of magma and the rocks that solidify from magma. Th us igneous petrologists are concerned with the entire spectrum of processes that describe how magmas are produced and how they ascend through the mantle and crust, their mineralogical and geochemical evolution, and their eruption or emplacement to form igneous rocks. Igneous petrology requires a working knowledge of mineralogy. Readers who wish to review the characteristics of the major rock-forming igneous minerals will fi nd a concise sum- mary in Appendix 1. Th e appendix emphasizes the identifi cation of rock-forming minerals in hand sample and in thin section. In addition, the appendix includes descriptions of minerals found in minor abundance but commonly occurring in igneous rocks, including accessory minerals that contain trace amounts of uranium and are important geochronometers. Before geologists can understand the origin of igneous rocks, they must classify and describe them. Th is chapter introduces the classifi cation of igneous rocks using the mineralogical classifi cation system recom- mended by the International Union of Geological Sciences (IUGS) Subcommission on the Systematics of Igneous Rocks, which has the advantage that it is relatively simple and can be applied in the fi eld. For rocks that are too fi ne-grained to name using this classifi cation, a geochemical classifi cation can be employed instead. Th e simplest of these, the total alkali versus silica classifi cation, is introduced in this text. -

Earth Sciences 3313A: IGNEOUS PETROLOGY ˗ Fall 2019
EARTH SCIENCES 3313A – Igneous Petrology Earth Sciences 3313A: IGNEOUS PETROLOGY ˗ Fall 2019 Arenal Volcano, Costa Rica Ultramafic flow, spinifex texture, fresh and altered Instructor: Robert Linnen Office B&GS 1000B email: [email protected] tel. ext. 89207 Meeting hours: There are no fixed office hours, please set up an appointment when you need to meet Teaching Assistant: to be determined Pre-requisites: ES 2206a/b - Mineral Systems, Crystallography and Optics Schedule Lectures: Tuesdays & Thursdays: 12:30-13:20, Room: NCB-295. Labs: Thursday 2:30-5:20 and, depending on enrolment, 6:00-9:00 Room: B&GS-1065 Important Dates Tuesday, October 17th: Midterm exam in class Fall Reading Week November 4-8 no classes Thursday, November 28: Lab exam in BGS 1065 December 8 – 19: Examination period. Final exam, date and location TBA. 1 EARTH SCIENCES 3313A – Igneous Petrology CALENDAR DESCRIPTION OF EARTH SCIENCES 3313A “IGNEOUS PETROLOGY" Study of igneous processes using rock and thin section descriptions (petrography). Discussion of how different compositions and conditions influence the phases present in a rock (phase equilibria). Association of different rock types with plate tectonic setting. WHAT ARE THE PRINCIPLE OBJECTIVES OF THIS COURSE? To outline the physical and chemical properties of magma, to introduce the techniques that are used to interpret the origin and evolution of different series of magmas and to examine in more detail magma evolution in specific igneous/tectonic environments. The laboratory is an integral part of the course and students will learn to identify common igneous rocks and textures in hand specimen and in thin section using a petrographic microscope. -

Quartz: a Bull's Eye on Optical Activity
Quartz: a Bull’s Eye on Optical Activity Elise A. Skalwold The Mineralogical Society of America William A. Bassett Title: Quartz: a Bull’s Eye on Optical Activity Authors: Elise Ann Skalwold & William Akers Bassett Edition: First edition Publisher: Mineralogical Society of America, Chantilly, Virginia, USA Copyright: © 2015 by the authors, artists, and photographers. Reproduced with permission. All Rights Reserved. ISBN: 978-0-939950-00-3 Photographer & Designer: Elise A. Skalwold Front cover: Natural quartz crystal 60 x 65 x 40 mm; Hot Springs, Arkansas; ex. Dr. R.W.M. Woodside collection. Back cover: Lab-grown quartz cluster, 140 mm x 90 mm (hydrothermally grown by Mila and Vladimir A. Klipov, R&D XTALS, Inc.). Below: Natural quartz crystals and basal sections. On-going collaboration with Cornell’s Professor Emeritus William A. Bassett is truly priceless to me for this and other projects in the wings, as well as for those over the past eight years of work and research together. Bill shares my enthusi- asm for exploring the fascinating aspects of the classical science of mineralogy, and as my co-author he sets the highest bar for accuracy. All students should be so lucky to have such a mentor. Elise A. Skalwold, 2015 Ithaca, New York Mineralogical Society of America Quartz: a Bull’s Eye on Optical Activity Elise A. Skalwold [email protected] William A. Bassett [email protected] Both Authors: Department of Earth & Atmospheric Sciences Snee Hall, Cornell University Ithaca, NY 14853 All photographs: Elise A. Skalwold Figure 1. The “bull’s eye” uniaxial optic figure characteristic of quartz is indicative of its optical activity. -

Optical Mineralogy in a Nutshell
Optical Mineralogy in a Nutshell Use of the petrographic microscope Slides borrowed/adapted from Jane Selverstone (University of New Mexico) and John Winter (Whitman College) Why use the petrographic microscope? • Identify minerals (no guessing!) • Determine rock type • Determine crystallization sequence • Document deformation history • Observe frozen-in reactions • Constrain P-T history • Note weathering/alteration • Fun, powerful, and cheap! The petrographic microscope Also called a polarizing microscope In order to use the scope, we need to understand a little about the physics of light, and then learn some tools and tricks… Polarized Light Microscopy Isotropic materials, which include gases, liquids, unstressed glasses and cubic crystals, demonstrate the same From Nikon optical properties in all directions. They have only one refractive index and no restriction on the vibration direction of light passing through them. Anisotropic materials, in contrast, which include 90 percent of all solid substances, have optical properties that vary with the orientation of incident light with the crystallographic axes. Anisotropic materials act as beam splitters and divide light rays into two parts. The technique of polarizing microscopy exploits the interference of the split light rays, as they are re-united along the same optical path to extract information about these materials. What happens as light moves through the scope? plane polarised light (single vibration direction) unpolarised light (all possible vibration directions) 1) Light passes -

Syllabus Optical Mineralogy
CRYSTALLOGRAPHY UNIT IV UNIT-IV: SYLLABUS OPTICAL MINERALOGY : Light: Corpuscular, electromagnetic and quantum theories. Ordinary light and plane polarized light. Refractive index an its determination: Relief method, Becke line, Central illumination, and Oblique illumination methods. Isotropism, isotropic minerals and isotropic ray velocity surface. Behaviour of light in isotropic minerals. Petrological Microscope and its parts-optical accessories and their uses:Quartz wedge, Gypsum plate and Mica plate. Study of Isotropic minerals using the petrological microscope: properties of isotropic minerals under parallel Nicol conditions. LightCorpuscular A source of light continuously emits tiny elastic particles called corpuscles. These particles or the corpuscles moves with high velocity as that of light and gets scattered in all directions of light. This theory says that the velocity of light changes with the change in density of the medium in which it is used. This theory could explain three main phenomena of light that is reflection, refraction, and rectilinear propagation of light. This theory also says that the color of light is dependent on the size of the corpuscles. Some of the main drawbacks of this theory are 1) This theory could not explain the phenomena of interference, diffraction, and polarization of light etc. 2) According to this theory the velocity of light in denser medium is greater than the velocity of light in rarer medium but this is proved wrong later 3) This theory assumes that the source of light looses the mass as it emits corpuscles; but not such determent in mass of the source of light is detected. 4) This theory proposes that velocity of the corpuscles increases as the temperature of the source increases as the temperature increases experiments have proved that the velocity of light is independent of temperature. -

GEOL 221 Mineralogy and Mineral Optics Course Syllabus
SAN DIEGO STATE UNIVERSITY GEOL 221 Mineralogy and Mineral Optics Course Syllabus Instructor: Professor David L. Kimbrough Email: [email protected], Phone: 594-1385 Lecture: MW 1000-1050 CSL 422, Lab: W 1400-1640, Lab study: M 1400-1640 CSL 425 Office: GMCS-229A; Office hours: MW 1100-1200; T 1115-1215; by appointment TA: Mark Nahabidian GMCS-133 TBA Course Prerequisite: Chem 200 or concurrent registration. Credit or concurrent registration in OCEAN 100 or GEOL 100 and 101 or GEOL 104 and 101; Geol 200; Required Texts: Introduction to Mineralogy, William D. Nesse, Oxford University Press. Minerals in Thin Section, Perkins & Henke, Prentice Hall Recommended Text: Dictionary of Geological Terms, AGI Bates & Jackson eds. or similar, The Complete Guide to Rocks & Minerals by John Farndon or similar Other required materials: Hand lens; calculator Classroom management: Attendance is crucial. Please let me know if you’re going to be absent for any reason. Be on time for class, don’t participate in excessive side-chatter or cause disruptions during class. Always respond to the instructor, the Teaching Assistant, and your fellow students in a respectful and civil manner. Cheating or plagiarism is not tolerated. It’s easy to spot and constitutes serious academic misconduct. Helpful hints to make Mineralogy easier and more fun! Attend class: Studies show that the most valuable time commitment by students in a course is the time actually spent in the classroom. Class time is the most important determinant of student success and yields the greatest improvement in student learning outcomes. Information is covered in class that is not in the textbook, and parts of the book are hard to understand. -
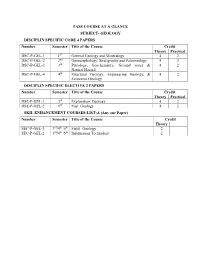
Pass Course at a Glance
PASS COURSE AT A GLANCE SUBJECT- GEOLOGY DISCIPLIN SPECIFIC CORE 4 PAPERS Number Semester Title of the Course Credit Theory Practical DSC-P-GEL-1 1ST General Geology and Mineralogy 4 2 DSC-P-GEL-2 2nd Geomorphology, Stratigraphy and Paleontology 4 2 DSC-P-GEL-3 3rd Petrology, Geochemistry, Ground water & 4 2 Natural Hazard DSC-P-GEL-4 4th Structural Geology, Engineering Geology, & 4 2 Economic Geology DISCIPLIN SPECIFIC ELECTIVE 2 PAPERS Number Semester Title of the Course Credit Theory Practical DSE-P-GEL-1 5th Exploration Geology 4 2 DSE-P-GEL-2 6th Fuel Geology 4 2 SKIL ENHANCEMENT COURSES-LIST-A (Any one Paper) Number Semester Title of the Course Credit Theory SEC-P-GEL-1 3rd/4th /5th Field Geology 2 SEC-P-GEL-2 3rd/4th /5th Information Technology 2 FIRST SEMESTER GEOLOGY PASS PAPER-I Theory Paper-I (General Geology, Crystallography and Mineralogy) Objectives of the Course: The aim of this course is to study General geology part can give an idea about endogenetic process operating inside the earth. study the crystals through external elements of symmetry, crystal classes and systems, and the relations of symmetry to the internal structure using the chemical and physical properties of the minerals. The course aims also to study the major mineral groups, their occurrences, physical, chemical and crystallographic properties and their possible uses in industry. In these units, the physical, chemical and optical properties of the minerals are described. One should know them to identify the types of rocks. Expected outcome: The said courses will make the students to understand about the interior of earth. -

Optical Mineralogy in a Nutshell
Optical Mineralogy in a Nutshell Use of the petrographic microscope in three easy lessons Part III © Jane Selverstone, University of New Mexico, 2003 A few new properties, and then some review… Cleavage – number and orientation of cleavage planes Twinning – type of twinning, orientation Extinction angle – parallel or inclined? Angle? Habit – characteristic form of mineral Cleavage Most easily observed in PPL (upper polarizer out), but visible in XN as well • No cleavages: quartz, olivine • 1 good cleavage: micas • 2 good cleavages: pyroxenes, amphiboles Cleavage 2 cleavages intersecting at ~90° pyroxene 120° 2 cleavages 60° intersecting at 60°/120°: amphibole Cleavage random fractures, no cleavage: olivine Twinning Presence and style of twinning can be diagnostic Twins are usually most obvious in XN (upper polarizer in) Twinning - some examples Clinopyroxene (augite) • Simple twin on {100} Plagioclase • Simple (Carlsbad) twin on (010) • Polysynthetic albite twins on (010) • Pericline twin on (h01) Extinction angle Extinction behavior is a function of the relationship between indicatrix orientation and crystallographic orientation parallel extinction inclined extinction Extinction angle – parallel extinction • All uniaxial minerals show parallel extinction • Orthorhombic minerals show parallel extinction (this is because the crystal axes and indicatrix axes coincide) orthopyroxene PPL XN Extinction angle - inclined extinction Monoclinic and triclinic minerals: indicatrix axes do not coincide with crystallographic axes These minerals have -

Magma Genesis, Plate Tectonics, and Chemical Differentiation of the Earth
REVIEWS OF GEOPHYSICS, VOL. 26, NO. 3, PAGES 370-404, AUGUST 1988 Magma Genesis, Plate Tectonics, and Chemical Differentiation of the Earth PETER J. WYLLIE Division of Geolo•7icaland Planetary Sciences,California Institute of Technolo•Ty,Pasadena Magma genesis,migration, and eruption have played prominent roles in the chemical differentiation of the Earth. Plate tectonics has provided the framework of tectonic environments for different suites of igneousrocks and the dynamic mechanismsfor moving massesof rock into melting regions.Petrology is rooted in geophysics.Petrological and geophysicalprocesses are calibrated by the phase equilibria of the materials. The geochemistry of basalts and mantle xenoliths demonstrates that the mantle is hetero- geneous.The geochemical reservoirs are related to mantle convection, with interpretation of a mantle layered or stratified or peppered with blobs. Seismic tomography is beginning to reveal the density distribution of the mantle in three dimensions,and together with fluid mechanical models and interpreta- tion of the geoid, closer limits are being placed on mantle convection. Petrological cross sectionscon- structed for various tectonic environments by transferring phase boundaries for source rocks onto assumedthermal structuresprovide physical frameworks for consideration of magmatic and metasoma- tic events,with examplesbeing given for basalts,andesites, and granites at ocean-continentconvergent plate boundaries, basalts and nephelinitesfrom a thermal plume beneath Hawaii, kimberlites in cratons, -

Short History of Teaching Mineralogy at the Eötvös Loránd University, Budapest
Acta Mineralogica-Petrographica, Szeged 2004, Vol. 45/1, pp. 5-20 1 SHORT HISTORY OF TEACHING MINERALOGY AT THE EÖTVÖS LORÁND UNIVERSITY, BUDAPEST 1 2 1 GY. BUDA , G. PAPP , T. G. WEISZBURG Megjegyzés: Kérem a teljes neveket! 1 Department of Mineralogy, Eötvös Loránd University, H-1117 Budapest, Pázmány Péter sétány 1/C, Hungary 2 Department of Mineralogy and Petrology, Hungarian Natural History Museum, H-1431Budapest. Pf.:137, Hungary e-mail: [email protected] We intend to overview the 230-year history of organised teaching of mineralogy at the Eötvös Loránd University. The University was founded in 1635. Students could learn certain elements of mineralogy already in the early period of the University within the frame of physics. Mineralogy, as an independent subject, has been part of the curriculum since 1774, the year when the Department of Natural History was founded. The separate Department of Mineralogy was established in 1849. While trying to divide the long historical span into periods, no unique concept appropriate in every respect was found, so changes of institutional structure, as well as the prominent mineralogy-related personalities are used as guidelines. For helping the reader not experienced in historical and cultural development of that part of Central Europe, at some points we are giving also explanatory notes related to political, cultural and science history. EARLY PERIOD OF TEACHING MINERALOGICAL KNOWLEDGE the Jesuit Ratio Studiorum (1599). Mineralogy was not (MINERALOGY AS PART OF PHYSICS, 1635–1774) included directly in the curriculum, but in books of some In 1635, when Péter Pázmány, archbishop of Esztergom, professors (e.g. -

Geology 1 Geology Courses GEOL 1100
Geology 1 Geology Courses GEOL 1100. Transitioning to University Studies in Geosciences. 1 Credit Hour (Lecture: 1 Hour, Lab: 1 Hour). An introduction to geosciences, including earth science, environmental science, geology, hydrogeology, and petroleum geology. Practical study designed to prepare the geoscience student for university life, aid in the development of skills for academic success, promote personal growth and responsibility, and encourage active involvement in the learning process. GEOL 1403. Physical Geology. 4 Credit Hours (Lecture: 3 Hours, Lab: 2 Hours). An introduction to the physical processes that operate in and on the planet Earth. Topics of discussion include: the Earth's structure, rocks and minerals, volcanoes, earthquakes, groundwater, rivers, glaciers, and deserts. Lab fee: $2. GEOL 1404. Historical Geology. 4 Credit Hours (Lecture: 3 Hours, Lab: 2 Hours). History of the Earth from the formation of the solar system to the present. Topics include the Earth's development, evolution of life on Earth, changes in the Earth's geography throughout its history, and the tools geologists use to investigate these topics. Lab fee $10. GEOL 1407. Introduction to Environmental Science. 4 Credit Hours (Lecture: 3 Hours, Lab: 2 Hours). Introduction to the study of the environment. The course will examine air, water, and soil pollution, and pollution remediation. Energy, mineral resources, and land use will be studied. The course will also emphasize a study of the water supply, water use, and water management. Much of the laboratory will focus on land use planning and environmental pollution remediation. Lab fee: $2. GEOL 1408. Natural Disasters. 4 Credit Hours (Lecture: 3 Hours, Lab: 2 Hours).