GEOL 221 Mineralogy and Mineral Optics Course Syllabus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

James Clerk Maxwell
James Clerk Maxwell JAMES CLERK MAXWELL Perspectives on his Life and Work Edited by raymond flood mark mccartney and andrew whitaker 3 3 Great Clarendon Street, Oxford, OX2 6DP, United Kingdom Oxford University Press is a department of the University of Oxford. It furthers the University’s objective of excellence in research, scholarship, and education by publishing worldwide. Oxford is a registered trade mark of Oxford University Press in the UK and in certain other countries c Oxford University Press 2014 The moral rights of the authors have been asserted First Edition published in 2014 Impression: 1 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, without the prior permission in writing of Oxford University Press, or as expressly permitted by law, by licence or under terms agreed with the appropriate reprographics rights organization. Enquiries concerning reproduction outside the scope of the above should be sent to the Rights Department, Oxford University Press, at the address above You must not circulate this work in any other form and you must impose this same condition on any acquirer Published in the United States of America by Oxford University Press 198 Madison Avenue, New York, NY 10016, United States of America British Library Cataloguing in Publication Data Data available Library of Congress Control Number: 2013942195 ISBN 978–0–19–966437–5 Printed and bound by CPI Group (UK) Ltd, Croydon, CR0 4YY Links to third party websites are provided by Oxford in good faith and for information only. -

Polarization Phenomena of Certain Fluorites
142 TIIE AMERICAN MINERALOGIST Pleochroic,e :colorless, ,:pale blue-green, It thus differs from the average vesuvianite only in slightly higher birefringence. The writer wishes to acknowledge his indebtedness to Col. Roebling, Mr. Gage, and Ward's Natural ScienceEstablishment for the privilege of examining specimensand to Dr. Larsen for the opticaldeterminations. POLARIZATION PHENOMENA OF CERTAIN FLUORITES A. L. PansoNs, Uniaersity oJ Toronto While determining the refractive index of fluorite by the method of minimum deviation the writer introduced a nicol prism before the telescopeof the goniometer to determine the plane of polarization of the refracted ray. On rotating the nicol no difference in the intensity of light could be observed,but on examining the reflected ray nearly complete polarization was obtained so that it was thought that the prism was not cut at the angle that should give polarization if Brewster's law applies to isometric crystals. A new prism was then cut from a crystal of fluorite from Madoc, Ontario, so that the angle between the reflected ray and the refracted ray should be ninety degrees. With this new prism the same phenomena were observed. A second nicol was now intro- duced between the collimator and the crystal so that the signal was extinguished. On rotating the two nicols simultaneously extinction was obtained throughout a complete rotation so that it would appear as though we may accept without question the state- ment that a ray of light passing through fluorite vibrates with equal facility in all directions at right angles to the direction of propagation and is not polarized. -

Quartz: a Bull's Eye on Optical Activity
Quartz: a Bull’s Eye on Optical Activity Elise A. Skalwold The Mineralogical Society of America William A. Bassett Title: Quartz: a Bull’s Eye on Optical Activity Authors: Elise Ann Skalwold & William Akers Bassett Edition: First edition Publisher: Mineralogical Society of America, Chantilly, Virginia, USA Copyright: © 2015 by the authors, artists, and photographers. Reproduced with permission. All Rights Reserved. ISBN: 978-0-939950-00-3 Photographer & Designer: Elise A. Skalwold Front cover: Natural quartz crystal 60 x 65 x 40 mm; Hot Springs, Arkansas; ex. Dr. R.W.M. Woodside collection. Back cover: Lab-grown quartz cluster, 140 mm x 90 mm (hydrothermally grown by Mila and Vladimir A. Klipov, R&D XTALS, Inc.). Below: Natural quartz crystals and basal sections. On-going collaboration with Cornell’s Professor Emeritus William A. Bassett is truly priceless to me for this and other projects in the wings, as well as for those over the past eight years of work and research together. Bill shares my enthusi- asm for exploring the fascinating aspects of the classical science of mineralogy, and as my co-author he sets the highest bar for accuracy. All students should be so lucky to have such a mentor. Elise A. Skalwold, 2015 Ithaca, New York Mineralogical Society of America Quartz: a Bull’s Eye on Optical Activity Elise A. Skalwold [email protected] William A. Bassett [email protected] Both Authors: Department of Earth & Atmospheric Sciences Snee Hall, Cornell University Ithaca, NY 14853 All photographs: Elise A. Skalwold Figure 1. The “bull’s eye” uniaxial optic figure characteristic of quartz is indicative of its optical activity. -

Optical Mineralogy in a Nutshell
Optical Mineralogy in a Nutshell Use of the petrographic microscope Slides borrowed/adapted from Jane Selverstone (University of New Mexico) and John Winter (Whitman College) Why use the petrographic microscope? • Identify minerals (no guessing!) • Determine rock type • Determine crystallization sequence • Document deformation history • Observe frozen-in reactions • Constrain P-T history • Note weathering/alteration • Fun, powerful, and cheap! The petrographic microscope Also called a polarizing microscope In order to use the scope, we need to understand a little about the physics of light, and then learn some tools and tricks… Polarized Light Microscopy Isotropic materials, which include gases, liquids, unstressed glasses and cubic crystals, demonstrate the same From Nikon optical properties in all directions. They have only one refractive index and no restriction on the vibration direction of light passing through them. Anisotropic materials, in contrast, which include 90 percent of all solid substances, have optical properties that vary with the orientation of incident light with the crystallographic axes. Anisotropic materials act as beam splitters and divide light rays into two parts. The technique of polarizing microscopy exploits the interference of the split light rays, as they are re-united along the same optical path to extract information about these materials. What happens as light moves through the scope? plane polarised light (single vibration direction) unpolarised light (all possible vibration directions) 1) Light passes -

Syllabus Optical Mineralogy
CRYSTALLOGRAPHY UNIT IV UNIT-IV: SYLLABUS OPTICAL MINERALOGY : Light: Corpuscular, electromagnetic and quantum theories. Ordinary light and plane polarized light. Refractive index an its determination: Relief method, Becke line, Central illumination, and Oblique illumination methods. Isotropism, isotropic minerals and isotropic ray velocity surface. Behaviour of light in isotropic minerals. Petrological Microscope and its parts-optical accessories and their uses:Quartz wedge, Gypsum plate and Mica plate. Study of Isotropic minerals using the petrological microscope: properties of isotropic minerals under parallel Nicol conditions. LightCorpuscular A source of light continuously emits tiny elastic particles called corpuscles. These particles or the corpuscles moves with high velocity as that of light and gets scattered in all directions of light. This theory says that the velocity of light changes with the change in density of the medium in which it is used. This theory could explain three main phenomena of light that is reflection, refraction, and rectilinear propagation of light. This theory also says that the color of light is dependent on the size of the corpuscles. Some of the main drawbacks of this theory are 1) This theory could not explain the phenomena of interference, diffraction, and polarization of light etc. 2) According to this theory the velocity of light in denser medium is greater than the velocity of light in rarer medium but this is proved wrong later 3) This theory assumes that the source of light looses the mass as it emits corpuscles; but not such determent in mass of the source of light is detected. 4) This theory proposes that velocity of the corpuscles increases as the temperature of the source increases as the temperature increases experiments have proved that the velocity of light is independent of temperature. -
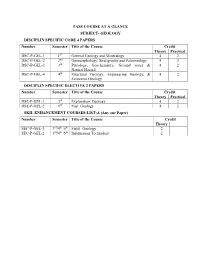
Pass Course at a Glance
PASS COURSE AT A GLANCE SUBJECT- GEOLOGY DISCIPLIN SPECIFIC CORE 4 PAPERS Number Semester Title of the Course Credit Theory Practical DSC-P-GEL-1 1ST General Geology and Mineralogy 4 2 DSC-P-GEL-2 2nd Geomorphology, Stratigraphy and Paleontology 4 2 DSC-P-GEL-3 3rd Petrology, Geochemistry, Ground water & 4 2 Natural Hazard DSC-P-GEL-4 4th Structural Geology, Engineering Geology, & 4 2 Economic Geology DISCIPLIN SPECIFIC ELECTIVE 2 PAPERS Number Semester Title of the Course Credit Theory Practical DSE-P-GEL-1 5th Exploration Geology 4 2 DSE-P-GEL-2 6th Fuel Geology 4 2 SKIL ENHANCEMENT COURSES-LIST-A (Any one Paper) Number Semester Title of the Course Credit Theory SEC-P-GEL-1 3rd/4th /5th Field Geology 2 SEC-P-GEL-2 3rd/4th /5th Information Technology 2 FIRST SEMESTER GEOLOGY PASS PAPER-I Theory Paper-I (General Geology, Crystallography and Mineralogy) Objectives of the Course: The aim of this course is to study General geology part can give an idea about endogenetic process operating inside the earth. study the crystals through external elements of symmetry, crystal classes and systems, and the relations of symmetry to the internal structure using the chemical and physical properties of the minerals. The course aims also to study the major mineral groups, their occurrences, physical, chemical and crystallographic properties and their possible uses in industry. In these units, the physical, chemical and optical properties of the minerals are described. One should know them to identify the types of rocks. Expected outcome: The said courses will make the students to understand about the interior of earth. -

Optical Mineralogy in a Nutshell
Optical Mineralogy in a Nutshell Use of the petrographic microscope in three easy lessons Part III © Jane Selverstone, University of New Mexico, 2003 A few new properties, and then some review… Cleavage – number and orientation of cleavage planes Twinning – type of twinning, orientation Extinction angle – parallel or inclined? Angle? Habit – characteristic form of mineral Cleavage Most easily observed in PPL (upper polarizer out), but visible in XN as well • No cleavages: quartz, olivine • 1 good cleavage: micas • 2 good cleavages: pyroxenes, amphiboles Cleavage 2 cleavages intersecting at ~90° pyroxene 120° 2 cleavages 60° intersecting at 60°/120°: amphibole Cleavage random fractures, no cleavage: olivine Twinning Presence and style of twinning can be diagnostic Twins are usually most obvious in XN (upper polarizer in) Twinning - some examples Clinopyroxene (augite) • Simple twin on {100} Plagioclase • Simple (Carlsbad) twin on (010) • Polysynthetic albite twins on (010) • Pericline twin on (h01) Extinction angle Extinction behavior is a function of the relationship between indicatrix orientation and crystallographic orientation parallel extinction inclined extinction Extinction angle – parallel extinction • All uniaxial minerals show parallel extinction • Orthorhombic minerals show parallel extinction (this is because the crystal axes and indicatrix axes coincide) orthopyroxene PPL XN Extinction angle - inclined extinction Monoclinic and triclinic minerals: indicatrix axes do not coincide with crystallographic axes These minerals have -

Short History of Teaching Mineralogy at the Eötvös Loránd University, Budapest
Acta Mineralogica-Petrographica, Szeged 2004, Vol. 45/1, pp. 5-20 1 SHORT HISTORY OF TEACHING MINERALOGY AT THE EÖTVÖS LORÁND UNIVERSITY, BUDAPEST 1 2 1 GY. BUDA , G. PAPP , T. G. WEISZBURG Megjegyzés: Kérem a teljes neveket! 1 Department of Mineralogy, Eötvös Loránd University, H-1117 Budapest, Pázmány Péter sétány 1/C, Hungary 2 Department of Mineralogy and Petrology, Hungarian Natural History Museum, H-1431Budapest. Pf.:137, Hungary e-mail: [email protected] We intend to overview the 230-year history of organised teaching of mineralogy at the Eötvös Loránd University. The University was founded in 1635. Students could learn certain elements of mineralogy already in the early period of the University within the frame of physics. Mineralogy, as an independent subject, has been part of the curriculum since 1774, the year when the Department of Natural History was founded. The separate Department of Mineralogy was established in 1849. While trying to divide the long historical span into periods, no unique concept appropriate in every respect was found, so changes of institutional structure, as well as the prominent mineralogy-related personalities are used as guidelines. For helping the reader not experienced in historical and cultural development of that part of Central Europe, at some points we are giving also explanatory notes related to political, cultural and science history. EARLY PERIOD OF TEACHING MINERALOGICAL KNOWLEDGE the Jesuit Ratio Studiorum (1599). Mineralogy was not (MINERALOGY AS PART OF PHYSICS, 1635–1774) included directly in the curriculum, but in books of some In 1635, when Péter Pázmány, archbishop of Esztergom, professors (e.g. -

Igneous Petrology 2001
MetamorphicMetamorphic PetrologyPetrology LectureLecture 1:1: MetamorphicMetamorphic phenomenaphenomena andand theirtheir characterization:characterization: AnAn introductionintroduction byby StephanStephan KK MatthäiMatthäi MP-SKM, slide 1 Course Objectives I will try to teach you: • To identify common metamorphic rocks in the field and infer their protoliths (original rock types and composition), • Understand how they formed, • Get broad estimates of the pressure and temperature conditions under which the rocks were metamorphosed, • How to use overprinting relationships and deformation structures to determine the geological / metamorphic history of the rocks, • Infer the burial depth and thermal history of the metamorphic pile, • Make PTt-path diagrams • Interpret the plate-tectonic setting of metamorphism, • Quantify the chemical changes that the rock underwent during metamorphism (gains & losses), Get you ready for independent field work. MP-SKM, slide 2 ES4.08 Prerequisites Geology: plate-tectonic settings, basics of sedimentary and igneous rocks, magmatism and volcanism (Internal Processes, Dynamic Earth) Mineralogy: ability to determine the main rock-forming minerals in hand specimen and thin section; ideally, a knowledge of the chemical composition of minerals (Minerals & Rocks, Optical Mineralogy & Petrography) Chemistry: stochiometry (balancing reactions), possible valency states of cations, law of mass action, equilibrium constants (Geochemistry 1) Thermodynamics: absolute basics – Gibbs free energy, heat capacity, entropy, -

B.Sc.( Srmester-3) Subject: Physics Course: US03CPHY01 Title: Optics
B.Sc.( Srmester-3) Subject: Physics Course: US03CPHY01 Title: Optics UNIT – III Polarization Introduction:- • Interference and diffraction phenomena proved that light is a wave motion. These phenomena are used to find wavelength of light. However, they do not give any indication regarding the character of waves. • Maxwell developed electromagnetic theory and suggested that light-waves are electromagnetic waves. Electromagnetic waves are transverse waves, so it is obvious that light waves are also transverse waves. • Longitudinal waves are waves in which particles of medium oscillate along the direction of propagation of wave (e.g. sound wave). • Transverse waves are waves in which particles of medium oscillate perpendicular to the direction of propagation of wave. (e.g. Electromagnetic waves.) • Polarization is possible in transverse wave. • Unpolarized Light is the light is which the planes of vibration are symmetrically distributed about the propagation direction of the wave. • Plane Polarized light is a wave in which the electric vector is everywhere confined to a single plane. • A linearly polarized light wave is a wave in which the electric vector oscillates in a given constant orientation. Production of Linearly Polarized Light: Linearly polarized light may be produced from unpolarized light using following optical phenomena. (i) Reflection (ii) Refraction (iii) Scattering (iv) Selective absorption (v) Double reflection. Polarized light has many important applications in industry and engineering. One of the most important applications is in liquid crystal displays (LCDs) which are widely used in wristwatches, calculators, T.V. Screens etc. An understanding of polarization is essential for understanding the propagation of electromagnetic waves guided through wave-guides and optical fibers. -

Chapter 8 Polarization
Phys 322 Chapter 8 Lecture 22 Polarization Reminder: Exam 2, October 22nd (see webpage) Dichroism = selective absorption of light of certain polarization Linear dichroism - selective absorption of one of the two P-state (linear) orthogonal polarizations Circular dichroism - selective absorption of L-state or R-state circular polarizations Using dichroic materials one can build a polarizer Dichroic crystals Anisotropic crystal structure: one polarization is absorbed more than the other Example: tourmaline Elastic constants for electrons may be different along two axes Polaroid 1928: dichroic sheet polarizer, J-sheet long tiny crystals of herapathite aligned in the plastic sheet Edwin Land 1938: H-sheet 1909-1991 Attach Iodine molecules to polymer molecules - molecular size iodine wires Presently produced: HN-38, HN-32, HN-22 Birefringence Elastic constants for electrons may be different along axes Resonance frequencies will be different for light polarized Refraction index depends on along different axes polarization: birefringence Dichroic crystal - absorbs one of the orthogonal P-states, transmits the other Optic axis of a crystal: the direction of linear polarization along which the resonance is different from the other two axes (assuming them equal) Calcite (CaCO3) Ca C O Image doubles Ordinary rays (o-rays) - unbent Extraordinary rays (e-rays) - bend Calcite (CaCO3) emerging rays are orthogonaly polarized Principal plane - any plane that contains optical axis Principal section - principal plane that is normal to one of the cleavage -

Optimized Morphologic Evaluation of Biostructures by Examination in Polarized Light and Differential Interference Contrast Microscopy
Rom J Leg Med [22] 275-282 [2014] DOI: 10.4323/rjlm.2014.275 © 2014 Romanian Society of Legal Medicine Optimized morphologic evaluation of biostructures by examination in polarized light and differential interference contrast microscopy Elena Patrascu1, Petru Razvan Melinte1, Gheorghe S. Dragoi2,* _________________________________________________________________________________________ Abstract: Differential interference contrast microscopy (DIC) was possible after the invention of Wollaston prisms that were modified by Nomarski and could duplicate linear polarized light before passing through a specimen (Condenser DIC prism) and to recompose it afterwards (Objective DIC prism), thus creating the phenomenon of interference that depends on the thickness and birefringence of the studied structures. The authors proposed themselves to discuss the performances of this microscope and to widen the area of its usage in the optimized microanatomic analysis of cells and tissues on the native or stained sections. The authors consider it necessary to use DIC microscopy both in forensic medicine (identification of diatoms) as well as in reproduction biology (spermogram) and in early discovery of uterine cervix cancer. Key Words: DIC microscopy, Wollaston prism, Nomarski prism, polarized light, interference. he progresses achieved in the area of wave (Maxwell, 1864) [2] and has a photogenic improvement and diversification of structure (Einstein, 1921) [3]. It consists of an electric T optic systems determined the increase of photonic and a magnetic field, orthogonal, which vibrate in microscopes performances regarding the phase contrast, phase, in the direction of propagation. Within an polarized light and not least, differential interference electromagnetic wave, the electric and the magnetic contrast (DIC) examinations.They assure nowadays fields oscillate simultaneously, but in different planes.