Mouse Rtl6 Knockout Project (CRISPR/Cas9)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Noncoding Rnas As Novel Pancreatic Cancer Targets
NONCODING RNAS AS NOVEL PANCREATIC CANCER TARGETS by Amy Makler A Thesis Submitted to the Faculty of The Charles E. Schmidt College of Science In Partial Fulfillment of the Requirements for the Degree of Master of Science Florida Atlantic University Boca Raton, FL August 2018 Copyright 2018 by Amy Makler ii ACKNOWLEDGEMENTS I would first like to thank Dr. Narayanan for his continuous support, constant encouragement, and his gentle, but sometimes critical, guidance throughout the past two years of my master’s education. His faith in my abilities and his belief in my future success ensured I continue down this path of research. Working in Dr. Narayanan’s lab has truly been an unforgettable experience as well as a critical step in my future endeavors. I would also like to extend my gratitude to my committee members, Dr. Binninger and Dr. Jia, for their support and suggestions regarding my thesis. Their recommendations added a fresh perspective that enriched our initial hypothesis. They have been indispensable as members of my committee, and I thank them for their contributions. My parents have been integral to my successes in life and their support throughout my education has been crucial. They taught me to push through difficulties and encouraged me to pursue my interests. Thank you, mom and dad! I would like to thank my boyfriend, Joshua Disatham, for his assistance in ensuring my writing maintained a logical progression and flow as well as his unwavering support. He was my rock when the stress grew unbearable and his encouraging words kept me pushing along. -

LDOC1 Expression in Fibroblasts of Patients with Down Syndrome
Open Life Sci. 2015; 10: 130–132 Communication Open Access Michele Salemi, Concetta Barone, Carmelo Romano, Salvatore Caniglia, Alda Ragalmuto, Francesco Scillato, Maria Grazia Salluzzo, Cataldo Scavuzzo, Mirella Vinci, Roberto Salluzzo, Corrado Romano, Paolo Bosco LDOC1 expression in fibroblasts of patients with Down syndrome Abstract: Down syndrome (DS) is characterised by intellectual disability and is caused by trisomy 21. Apoptosis 1 Introduction is a programmed cell death process and is involved in Down syndrome (DS) is the most common human genetic neurodegenerative diseases such as Alzheimer. People disorder affecting 1 in every 700-800 live births [1]. More with DS can develop some traits of Alzheimer disease at an than 99% of the individuals with DS have an extra copy earlier age than subjects without trisomy 21. The leucine of the entire chromosome 21, 95% of which are free zipper, down regulated in cancer 1 (LDOC1) appears to be trisomies and the remainder are mosaics or result from involved in the apoptotic pathways. The aim of the present translocations [2,3]. The phenotype of Down syndrome work was to detect the presence of intracellular synthesis is characterized by distinctive physical phenotype, of LDOC1 protein and LDOC1 mRNA in fibroblast cultures hypotonia, immunological defects, endocardial, from DS subjects. The western blot shows the presence of hematological and endocrinal alterations, intellectual LDOC1 protein in fibroblasts of DS subjects but no evidence disability, and behavioral and cognitive deficits [4]. of LDOC1 protein in fibroblasts of normal subjects. LDOC1 The leucine zipper down regulated in cancer 1 (LDOC1) gene mRNA expression is increased in fibroblasts from DS gene was mapped at Xq27 and consists of only one exon subjects compared to fibroblasts from normal subjects. -

Salivary LDOC1 Is a Gender-Difference Biomarker of Oral Squamous Cell Carcinoma
Salivary LDOC1 is a gender-difference biomarker of oral squamous cell carcinoma Chung-Ji Liu1,2,3,4,*, Jen-Hao Chen5,6,*, Shih-Min Hsia7, Chiu-Chu Liao8, Hui-Wen Chang8, Tzong-Ming Shieh9 and Yin-Hwa Shih8 1 Department of Oral and Maxillofacial Surgery, MacKay Memorial Hospital, Taipei, Taiwan 2 MacKay Medical College, Taipei, Taiwan 3 Department of Medical Research, MacKay Memorial Hospital, Taipei, Taiwan 4 Institute of Oral Biology, School of Dentistry, National Yang-Ming University, Taipei, Taiwan 5 School of Dentistry, College of Dental Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan 6 Prosthodontics Department, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan 7 School of Nutrition and Health Sciences, College of Nutrition, Taipei Medical University, Taipei, Taiwan 8 Department of Healthcare Administration, Asia University, Taichung, Taiwan 9 Department of Dental Hygiene, College of Health Care, China Medical University, Taichung, Taiwan * These authors contributed equally to this work. ABSTRACT Background. The X-linked tumor suppressor gene LDOC1 is reported to be involved in oral cancer. The detection of biomarkers in salivary RNA is a non-invasive strategy for diagnosing many diseases. The aim of the present study was to investigate the potential of salivary LDOC1 as a biomarker of oral cancer. Methods. We determined the expression levels of LDOC1 in the saliva of oral squamous cell carcinoma (OSCC) subjects, and investigated its correlation with various clinico- pathological characteristics. The expression levels of salivary LDOC1 were detected in 53 OSCC subjects and 43 healthy controls using quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis. We used Fisher's exact test to analyze Submitted 31 January 2019 the correlations between expression levels and clinicopathological characteristics. -

Design, Construction and Implementation of a Web-Based Database System for Tumor Suppressor Genes
DESIGN, CONSTRUCTION AND IMPLEMENTATION OF A WEB-BASED DATABASE SYSTEM FOR TUMOR SUPPRESSOR GENES By YANMING YANG A THESIS PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE UNIVERSITY OF FLORIDA 2003 Copyright 2003 by Yanming Yang ACKNOWLEDGMENTS I would like to express my gratitude to Dr. Li M. Fu, my major adviser, for his guidance in the establishment of the research project and advice on my study progress. My thanks also go to Dr. Mark Yang of the Department of Statistics and Dr. Donald McCarty of the Department of Horticultural Science for serving as my committee members and their suggestions for finalizing the thesis. I would like to thank my wife, Xidan Zhou, and my daughters, JingRu and Kathleen, for their support to my personal life, their encouragement to my studies, and their sharing of frustration and happiness with me. iii TABLE OF CONTENTS Page ACKNOWLEDGMENTS ................................................................................................. iii TABLE OF CONTENTS................................................................................................... iv LIST OF FIGURES ........................................................................................................... vi ABSTRACT...................................................................................................................... vii CHAPTER 1 INTRODUCTION ...........................................................................................................1 -

Spatial Maps of Prostate Cancer Transcriptomes Reveal an Unexplored Landscape of Heterogeneity
ARTICLE DOI: 10.1038/s41467-018-04724-5 OPEN Spatial maps of prostate cancer transcriptomes reveal an unexplored landscape of heterogeneity Emelie Berglund1, Jonas Maaskola1, Niklas Schultz2, Stefanie Friedrich3, Maja Marklund1, Joseph Bergenstråhle1, Firas Tarish2, Anna Tanoglidi4, Sanja Vickovic 1, Ludvig Larsson1, Fredrik Salmeń1, Christoph Ogris3, Karolina Wallenborg2, Jens Lagergren5, Patrik Ståhl1, Erik Sonnhammer3, Thomas Helleday2 & Joakim Lundeberg 1 1234567890():,; Intra-tumor heterogeneity is one of the biggest challenges in cancer treatment today. Here we investigate tissue-wide gene expression heterogeneity throughout a multifocal prostate cancer using the spatial transcriptomics (ST) technology. Utilizing a novel approach for deconvolution, we analyze the transcriptomes of nearly 6750 tissue regions and extract distinct expression profiles for the different tissue components, such as stroma, normal and PIN glands, immune cells and cancer. We distinguish healthy and diseased areas and thereby provide insight into gene expression changes during the progression of prostate cancer. Compared to pathologist annotations, we delineate the extent of cancer foci more accurately, interestingly without link to histological changes. We identify gene expression gradients in stroma adjacent to tumor regions that allow for re-stratification of the tumor micro- environment. The establishment of these profiles is the first step towards an unbiased view of prostate cancer and can serve as a dictionary for future studies. 1 Department of Gene Technology, School of Engineering Sciences in Chemistry, Biotechnology and Health, Royal Institute of Technology (KTH), Science for Life Laboratory, Tomtebodavägen 23, Solna 17165, Sweden. 2 Department of Oncology-Pathology, Karolinska Institutet (KI), Science for Life Laboratory, Tomtebodavägen 23, Solna 17165, Sweden. 3 Department of Biochemistry and Biophysics, Stockholm University, Science for Life Laboratory, Tomtebodavägen 23, Solna 17165, Sweden. -
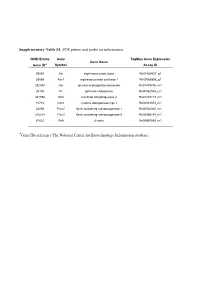
Supplementary Table S1. PCR Primer and Probe Set Information
Supplementary Table S1. PCR primer and probe set information NCBI Entrez Gene TaqMan Gene Expression Gene Name Gene ID* Symbol Assay ID 59085 Asl argininosuccinate lyase Rn01480437_g1 25698 Ass1 argininosuccinate synthase 1 Rn00565808_g1 292804 Gpi glucose-6-phosphate isomerase Rn01475756_m1 25104 Pc pyruvate carboxylase Rn00562534_m1 361596 Idh2 isocitrate dehydrogenase 2 Rn01478119_m1 81718 Cdo1 cysteine dioxygenasetype 1 Rn00583853_m1 25256 Fmo1 flavin containing monooxygenase 1 Rn00562945_m1 246248 Fmo5 flavin containing monooxygenase 5 Rn00595199_m1 81822 Actb β-actin Rn00667869_m1 *Gene IDs reference The National Center for Biotechnology Information database 1 Supplementary Table S2. Metabolite assignments and H NMR data in urine samples of rat* Urine Metabolite δ1H (ppm) 1 N-Methylnicotinamide 4.48(s) 8.18(t) 8.90(d) 8.97(d) 2 Nicotinamide N-oxide 7.73(dd) 8.12(m) 8.48(m) 3 Formate 8.46(s) 4 Hippurate 3.97(d) 7.55(t) 7.64(m) 7.84(dd) 8.56(s) 5 Tryptophan 3.30(dd) 3.47(dd) 4.05(dd) 7.20(m) 7.28(m) 7.50(d) 7.71(d) 6 Phenylalanine 3.11(dd) 3.28(dd) 3.99(dd) 7.36(m) 7.42(m) 7 Tyrosine 3.04(dd) 3.19(dd) 3.93(dd) 6.89(m) 7.17(m) 8 Fumarate 6.53(s) 9 Allantoin 5.39(s) 6.05(s) 7.26(s) 8.01(s) 10 Urea 5.80(s) 11 Glucose 3.24(dd) 3.39(t) 3.40(t) 3.45(m) 3.48(t) 3.53(dd) 3.70(t) 3.72(dd) 3.76(dd) 3.82(m) 3.84(dd) 3.89(dd) 4.65(d) 5.24(d) 12 Tartrate 4.36(s) 13 Threonine 1.33(d) 3.58(d) 4.24(m) 14 Creatinine 3.05(s) 4.06(s) 15 Creatine 3.02(s) 3.95(s) 16 Glycine 3.57(s) 17 Taurine 3.27(t) 3.43(t) 18 Choline 3.20(s) 3.51(m) 4.06(m) 19 cis-Aconitate -

Predict AID Targeting in Non-Ig Genes Multiple Transcription Factor
Downloaded from http://www.jimmunol.org/ by guest on September 26, 2021 is online at: average * The Journal of Immunology published online 20 March 2013 from submission to initial decision 4 weeks from acceptance to publication Multiple Transcription Factor Binding Sites Predict AID Targeting in Non-Ig Genes Jamie L. Duke, Man Liu, Gur Yaari, Ashraf M. Khalil, Mary M. Tomayko, Mark J. Shlomchik, David G. Schatz and Steven H. Kleinstein J Immunol http://www.jimmunol.org/content/early/2013/03/20/jimmun ol.1202547 Submit online. Every submission reviewed by practicing scientists ? is published twice each month by http://jimmunol.org/subscription Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts http://www.jimmunol.org/content/suppl/2013/03/20/jimmunol.120254 7.DC1 Information about subscribing to The JI No Triage! Fast Publication! Rapid Reviews! 30 days* Why • • • Material Permissions Email Alerts Subscription Supplementary The Journal of Immunology The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2013 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. This information is current as of September 26, 2021. Published March 20, 2013, doi:10.4049/jimmunol.1202547 The Journal of Immunology Multiple Transcription Factor Binding Sites Predict AID Targeting in Non-Ig Genes Jamie L. Duke,* Man Liu,†,1 Gur Yaari,‡ Ashraf M. Khalil,x Mary M. Tomayko,{ Mark J. Shlomchik,†,x David G. -

Identification of Novel Regulatory Genes in Acetaminophen
IDENTIFICATION OF NOVEL REGULATORY GENES IN ACETAMINOPHEN INDUCED HEPATOCYTE TOXICITY BY A GENOME-WIDE CRISPR/CAS9 SCREEN A THESIS IN Cell Biology and Biophysics and Bioinformatics Presented to the Faculty of the University of Missouri-Kansas City in partial fulfillment of the requirements for the degree DOCTOR OF PHILOSOPHY By KATHERINE ANNE SHORTT B.S, Indiana University, Bloomington, 2011 M.S, University of Missouri, Kansas City, 2014 Kansas City, Missouri 2018 © 2018 Katherine Shortt All Rights Reserved IDENTIFICATION OF NOVEL REGULATORY GENES IN ACETAMINOPHEN INDUCED HEPATOCYTE TOXICITY BY A GENOME-WIDE CRISPR/CAS9 SCREEN Katherine Anne Shortt, Candidate for the Doctor of Philosophy degree, University of Missouri-Kansas City, 2018 ABSTRACT Acetaminophen (APAP) is a commonly used analgesic responsible for over 56,000 overdose-related emergency room visits annually. A long asymptomatic period and limited treatment options result in a high rate of liver failure, generally resulting in either organ transplant or mortality. The underlying molecular mechanisms of injury are not well understood and effective therapy is limited. Identification of previously unknown genetic risk factors would provide new mechanistic insights and new therapeutic targets for APAP induced hepatocyte toxicity or liver injury. This study used a genome-wide CRISPR/Cas9 screen to evaluate genes that are protective against or cause susceptibility to APAP-induced liver injury. HuH7 human hepatocellular carcinoma cells containing CRISPR/Cas9 gene knockouts were treated with 15mM APAP for 30 minutes to 4 days. A gene expression profile was developed based on the 1) top screening hits, 2) overlap with gene expression data of APAP overdosed human patients, and 3) biological interpretation including assessment of known and suspected iii APAP-associated genes and their therapeutic potential, predicted affected biological pathways, and functionally validated candidate genes. -

FULLTEXT01.Pdf
To identify novel oncogenes for the design of novel tools for diagnosis and treatment of cancer Master Degree Project in infection Biology 60 ECTS Spring term Year Selva Kumar Mandiramoorthy [email protected] 19930713-3513 Course responsible Diana Tilvek [email protected] School of Bio science University of skÖvde, Sweden Examiner Patric Nilsson [email protected] School of Bio-science University of skÖvde, Sweden Supervisor Annica K. B. Gad, Ph.D. Senior/ Leading Researcher (R4) [email protected] CQM- Madeira Chemistry Research Centre / Centro de Química da Madeira University of Madeira Campus da Penteada 9020-105 Funchal, PORTUGAL ABSTRACT Cancer is a disease caused by an uncontrolled cell growth that destroys the healthy tissue of the body. It is one of the deadliest diseases in the world that alters many cellular mechanisms and features. In this report, a list of 22 upregulated oncogenes is studied to identify the novel oncogene. The need to determine the novel oncogene is to develop the anti-cancer agent. To determine the novel oncogene, gene enrichment analysis (GEA) was performed. It is a method to identify classes of genes that are over-represented in the large set of genes to determine the phenotypes of the organisms. DAVID and PANTHER are the methods used to carry on this study as it has Gene Ontology (GO) embedded in it. The GEA uses fishers exact test to determine the enriched gene by the standard p-value of 0.05. To further study the oncogene Network Enrichment Analysis was performed with EVINET. We found that microtubule was significantly enriched in NEA. -

Integrated Analysis of Mutations, Mirna and Mrna Expression In
Dong et al. BMC Systems Biology 2010, 4:163 http://www.biomedcentral.com/1752-0509/4/163 RESEARCH ARTICLE Open Access Integrated analysis of mutations, miRNA and mRNA expression in glioblastoma Hua Dong1,2,LiLuo2, Shengjun Hong1, Hoicheong Siu1, Yanghua Xiao3, Li Jin1, Rui Chen4, Momiao Xiong1,2* Abstract Background: Glioblastoma arises from complex interactions between a variety of genetic alterations and environmental perturbations. Little attention has been paid to understanding how genetic variations, altered gene expression and microRNA (miRNA) expression are integrated into networks which act together to alter regulation and finally lead to the emergence of complex phenotypes and glioblastoma. Results: We identified association of somatic mutations in 14 genes with glioblastoma, of which 8 genes are newly identified, and association of loss of heterozygosity (LOH) is identified in 11 genes with glioblastoma, of which 9 genes are newly discovered. By gene coexpression network analysis, we indentified 15 genes essential to the function of the network, most of which are cancer related genes. We also constructed miRNA coexpression networks and found 19 important miRNAs of which 3 were significantly related to glioblastoma patients’ survival. We identified 3,953 predicted miRNA-mRNA pairs, of which 14 were previously verified by experiments in other groups. Using pathway enrichment analysis we also found that the genes in the target network of the top 19 important miRNAs were mainly involved in cancer related signaling pathways, synaptic transmission and nervous systems processes. Finally, we developed new methods to decipher the pathway connecting mutations, expression information and glioblastoma. We indentified 4 cis-expression quantitative trait locus (eQTL): TP53, EGFR, NF1 and PIK3C2G; 262 trans eQTL and 26 trans miRNA eQTL for somatic mutation; 2 cis-eQTL: NRAP and EGFR; 409 trans- eQTL and 27 trans- miRNA eQTL for lost of heterozygosity (LOH) mutation. -

Leucine Zipper, Down Regulated in Cancer‑1 Gene Expression in Prostate Cancer
2796 ONCOLOGY LETTERS 12: 2796-2800, 2016 Leucine zipper, down regulated in cancer‑1 gene expression in prostate cancer MICHELE SALEMI1, NUNZIATA BARONE2, SANDRO LA VIGNERA2, ROSITA A. CONDORELLI2, DOMENICO RECUPERO2, ANTONIO GALIA3, FILIPPO FRAGGETTA3, ANNA MARIA AIELLO3, PIETRO PEPE4, ROBERTO CASTIGLIONE2, ENZO VICARI2 and ALDO E. CALOGERO2 1Laboratory of Cytogenetics, National Institute for Research and Treatment Oasi Maria SS, Institute for Research on Mental Retardation and Brain Aging, I-94018 Troina; 2Department of Clinical and Experimental Medicine, Section of Endocrinology, Andrology and Internal Medicine, University of Catania, I-95123 Catania; 3Pathology and 4Urology Units, Cannizzaro Hospital, I-95100 Catania, Italy Received April 8, 2015; Accepted June 12, 2016 DOI: 10.3892/ol.2016.4983 Abstract. Numerous genetic alterations have been implicated Introduction in the development of prostate cancer (PCa). DNA and protein microarrays have enabled the identification of genes associ- Prostate cancer (PCa) is a slow-growing but often aggressive ated with apoptosis, which is important in PCa development. cancer (1). The frequency of PCa is particularly high in Europe Despite the molecular mechanisms are not entirely understood, and USA (2,3). Factors such as diet, habit and genetic back- inhibition of apoptosis is a critical pathophysiological factor that ground have been implicated in the development of PCa (4). contributes to the onset and progression of PCa. Leucine zipper, The genetic background may contribute to the risk of PCa, as down-regulated in cancer 1 (LDOC-1) is a known regulator of suggested by associations with race, family and specific gene the nuclear factor (NF)-mediated pathway of apoptosis through variants (5).