Wildfowl 25 EDITED by G
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Endemic Ducks of Remote Islands DAVID LACK Introduction with the Names and Measurements of the Resident Forms of Anas
Ducks of remote islands 5 The endemic ducks of remote islands DAVID LACK Introduction with the names and measurements of the resident forms of Anas. On large islands This paper is concerned with all those and on islands near continents many ducks which have formed distinctive sub species of ducks coexist. For instance, 13 species restricted to one particular small species, 7 in the genus Anas, breed regu and remote island or archipelago. Only larly in Britain (Parslow 1967), 16 species, dabbling ducks in the genus Anas are 6 in the genus Anas, in Iceland (F. involved. Many species in this genus have Gudmundsson pers, com.), 8, 5 in the an extremely wide range, but the average genus Anas, in the Falkland Islands number of subspecies per species is as (Cawkell and Hamilton 1961) and 7, with low as 1.9 for the 38 species recognised 2 more in the nineteenth century, 4 in the by Delacour (1954-64). Hence the fact genus Anas, in New Zealand (Williams that nearly all the ducks on remote small 1964). Some of the Falklands and New islands are separate subspecies means that, Zealand forms are endemic, not so those for ducks, they belong to relatively of British or Iceland. Although the isolated populations. In contrast, on some various species of ducks look superficially of the archipelagos concerned, notably alike, especially when seen together in a the Hawaiian Islands and Galapagos, collection of living waterfowl, they have various of the land birds are distinctive in fact evolved an adaptive radiation species or even genera. -

South Georgia and Antarctic Odyssey
South Georgia and Antarctic Odyssey 30 November – 18 December 2019 | Greg Mortimer About Us Aurora Expeditions embodies the spirit of adventure, travelling to some of the most wild opportunity for adventure and discovery. Our highly experienced expedition team of and remote places on our planet. With over 28 years’ experience, our small group voyages naturalists, historians and destination specialists are passionate and knowledgeable – they allow for a truly intimate experience with nature. are the secret to a fulfilling and successful voyage. Our expeditions push the boundaries with flexible and innovative itineraries, exciting Whilst we are dedicated to providing a ‘trip of a lifetime’, we are also deeply committed to wildlife experiences and fascinating lectures. You’ll share your adventure with a group education and preservation of the environment. Our aim is to travel respectfully, creating of like-minded souls in a relaxed, casual atmosphere while making the most of every lifelong ambassadors for the protection of our destinations. DAY 1 | Saturday 30 November 2019 Ushuaia, Beagle Channel Position: 20:00 hours Course: 83° Wind Speed: 20 knots Barometer: 991 hPa & steady Latitude: 54°49’ S Wind Direction: W Air Temp: 6° C Longitude: 68°18’ W Sea Temp: 5° C Explore. Dream. Discover. —Mark Twain in the soft afternoon light. The wildlife bonanza was off to a good start with a plethora of seabirds circling the ship as we departed. Finally we are here on the Beagle Channel aboard our sparkling new ice-strengthened vessel. This afternoon in the wharf in Ushuaia we were treated to a true polar welcome, with On our port side stretched the beech forested slopes of Argentina, while Chile, its mountain an invigorating breeze sweeping the cobwebs of travel away. -

South Georgia & Antarctic Odyssey
South Georgia & Antarctic Odyssey 16 January – 02 February 2019 | Polar Pioneer About Us Aurora Expeditions embodies the spirit of adventure, travelling to some of the most wild and adventure and discovery. Our highly experienced expedition team of naturalists, historians and remote places on our planet. With over 27 years’ experience, our small group voyages allow for destination specialists are passionate and knowledgeable – they are the secret to a fulfilling a truly intimate experience with nature. and successful voyage. Our expeditions push the boundaries with flexible and innovative itineraries, exciting wildlife Whilst we are dedicated to providing a ‘trip of a lifetime’, we are also deeply committed to experiences and fascinating lectures. You’ll share your adventure with a group of like-minded education and preservation of the environment. Our aim is to travel respectfully, creating souls in a relaxed, casual atmosphere while making the most of every opportunity for lifelong ambassadors for the protection of our destinations. DAY 1 | Wednesday 16 January 2019 Ushuaia; Beagle Channel Position: 19:38 hours Course: 106° Wind Speed: 12 knots Barometer: 1006.6 hPa & steady Latitude: 54° 51’ S Speed: 12 knots Wind Direction: W Air Temp: 11°C Longitude: 68° 02’ W Sea Temp: 7°C The land was gone, all but a little streak, away off on the edge of the water, and We explored the decks, ventured down to the dining rooms for tea and coffee, then climbed down under us was just ocean, ocean, ocean—millions of miles of it, heaving up and down the various staircases. Howard then called us together to introduce the Aurora team and give a lifeboat and safety briefing. -

Disaggregation of Bird Families Listed on Cms Appendix Ii
Convention on the Conservation of Migratory Species of Wild Animals 2nd Meeting of the Sessional Committee of the CMS Scientific Council (ScC-SC2) Bonn, Germany, 10 – 14 July 2017 UNEP/CMS/ScC-SC2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II (Prepared by the Appointed Councillors for Birds) Summary: The first meeting of the Sessional Committee of the Scientific Council identified the adoption of a new standard reference for avian taxonomy as an opportunity to disaggregate the higher-level taxa listed on Appendix II and to identify those that are considered to be migratory species and that have an unfavourable conservation status. The current paper presents an initial analysis of the higher-level disaggregation using the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World Volumes 1 and 2 taxonomy, and identifies the challenges in completing the analysis to identify all of the migratory species and the corresponding Range States. The document has been prepared by the COP Appointed Scientific Councilors for Birds. This is a supplementary paper to COP document UNEP/CMS/COP12/Doc.25.3 on Taxonomy and Nomenclature UNEP/CMS/ScC-Sc2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II 1. Through Resolution 11.19, the Conference of Parties adopted as the standard reference for bird taxonomy and nomenclature for Non-Passerine species the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World, Volume 1: Non-Passerines, by Josep del Hoyo and Nigel J. Collar (2014); 2. -
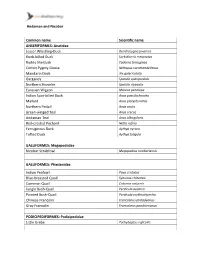
Andaman and Nicobar Common Name Scientific Name
Andaman and Nicobar Common name Scientific name ANSERIFORMES: Anatidae Lesser Whistling-Duck Dendrocygna javanica Knob-billed Duck Sarkidiornis melanotos Ruddy Shelduck Tadorna ferruginea Cotton Pygmy-Goose Nettapus coromandelianus Mandarin Duck Aix galericulata Garganey Spatula querquedula Northern Shoveler Spatula clypeata Eurasian Wigeon Mareca penelope Indian Spot-billed Duck Anas poecilorhyncha Mallard Anas platyrhynchos Northern Pintail Anas acuta Green-winged Teal Anas crecca Andaman Teal Anas albogularis Red-crested Pochard Netta rufina Ferruginous Duck Aythya nyroca Tufted Duck Aythya fuligula GALLIFORMES: Megapodiidae Nicobar Scrubfowl Megapodius nicobariensis GALLIFORMES: Phasianidae Indian Peafowl Pavo cristatus Blue-breasted Quail Synoicus chinensis Common Quail Coturnix coturnix Jungle Bush-Quail Perdicula asiatica Painted Bush-Quail Perdicula erythrorhyncha Chinese Francolin Francolinus pintadeanus Gray Francolin Francolinus pondicerianus PODICIPEDIFORMES: Podicipedidae Little Grebe Tachybaptus ruficollis Andaman and Nicobar COLUMBIFORMES: Columbidae Rock Pigeon Columba livia Andaman Wood-Pigeon Columba palumboides Eurasian Collared-Dove Streptopelia decaocto Red Collared-Dove Streptopelia tranquebarica Spotted Dove Streptopelia chinensis Laughing Dove Streptopelia senegalensis Andaman Cuckoo-Dove Macropygia rufipennis Asian Emerald Dove Chalcophaps indica Nicobar Pigeon Caloenas nicobarica Andaman Green-Pigeon Treron chloropterus Green Imperial-Pigeon Ducula aenea Nicobar Imperial-Pigeon Ducula nicobarica Pied Imperial-Pigeon -

A 2010 Supplement to Ducks, Geese, and Swans of the World
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Ducks, Geese, and Swans of the World by Paul A. Johnsgard Papers in the Biological Sciences 2010 The World’s Waterfowl in the 21st Century: A 2010 Supplement to Ducks, Geese, and Swans of the World Paul A. Johnsgard University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/biosciducksgeeseswans Part of the Ornithology Commons Johnsgard, Paul A., "The World’s Waterfowl in the 21st Century: A 2010 Supplement to Ducks, Geese, and Swans of the World" (2010). Ducks, Geese, and Swans of the World by Paul A. Johnsgard. 20. https://digitalcommons.unl.edu/biosciducksgeeseswans/20 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Ducks, Geese, and Swans of the World by Paul A. Johnsgard by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. The World’s Waterfowl in the 21st Century: A 200 Supplement to Ducks, Geese, and Swans of the World Paul A. Johnsgard Pages xvii–xxiii: recent taxonomic changes, I have revised sev- Introduction to the Family Anatidae eral of the range maps to conform with more current information. For these updates I have Since the 978 publication of my Ducks, Geese relied largely on Kear (2005). and Swans of the World hundreds if not thou- Other important waterfowl books published sands of publications on the Anatidae have since 978 and covering the entire waterfowl appeared, making a comprehensive literature family include an identification guide to the supplement and text updating impossible. -

Antarctic Primer
Antarctic Primer By Nigel Sitwell, Tom Ritchie & Gary Miller By Nigel Sitwell, Tom Ritchie & Gary Miller Designed by: Olivia Young, Aurora Expeditions October 2018 Cover image © I.Tortosa Morgan Suite 12, Level 2 35 Buckingham Street Surry Hills, Sydney NSW 2010, Australia To anyone who goes to the Antarctic, there is a tremendous appeal, an unparalleled combination of grandeur, beauty, vastness, loneliness, and malevolence —all of which sound terribly melodramatic — but which truly convey the actual feeling of Antarctica. Where else in the world are all of these descriptions really true? —Captain T.L.M. Sunter, ‘The Antarctic Century Newsletter ANTARCTIC PRIMER 2018 | 3 CONTENTS I. CONSERVING ANTARCTICA Guidance for Visitors to the Antarctic Antarctica’s Historic Heritage South Georgia Biosecurity II. THE PHYSICAL ENVIRONMENT Antarctica The Southern Ocean The Continent Climate Atmospheric Phenomena The Ozone Hole Climate Change Sea Ice The Antarctic Ice Cap Icebergs A Short Glossary of Ice Terms III. THE BIOLOGICAL ENVIRONMENT Life in Antarctica Adapting to the Cold The Kingdom of Krill IV. THE WILDLIFE Antarctic Squids Antarctic Fishes Antarctic Birds Antarctic Seals Antarctic Whales 4 AURORA EXPEDITIONS | Pioneering expedition travel to the heart of nature. CONTENTS V. EXPLORERS AND SCIENTISTS The Exploration of Antarctica The Antarctic Treaty VI. PLACES YOU MAY VISIT South Shetland Islands Antarctic Peninsula Weddell Sea South Orkney Islands South Georgia The Falkland Islands South Sandwich Islands The Historic Ross Sea Sector Commonwealth Bay VII. FURTHER READING VIII. WILDLIFE CHECKLISTS ANTARCTIC PRIMER 2018 | 5 Adélie penguins in the Antarctic Peninsula I. CONSERVING ANTARCTICA Antarctica is the largest wilderness area on earth, a place that must be preserved in its present, virtually pristine state. -

Contents Contents
Traveler’s Guide WILDLIFE WATCHINGTraveler’s IN PERU Guide WILDLIFE WATCHING IN PERU CONTENTS CONTENTS PERU, THE NATURAL DESTINATION BIRDS Northern Region Lambayeque, Piura and Tumbes Amazonas and Cajamarca Cordillera Blanca Mountain Range Central Region Lima and surrounding areas Paracas Huánuco and Junín Southern Region Nazca and Abancay Cusco and Machu Picchu Puerto Maldonado and Madre de Dios Arequipa and the Colca Valley Puno and Lake Titicaca PRIMATES Small primates Tamarin Marmosets Night monkeys Dusky titi monkeys Common squirrel monkeys Medium-sized primates Capuchin monkeys Saki monkeys Large primates Howler monkeys Woolly monkeys Spider monkeys MARINE MAMMALS Main species BUTTERFLIES Areas of interest WILD FLOWERS The forests of Tumbes The dry forest The Andes The Hills The cloud forests The tropical jungle www.peru.org.pe [email protected] 1 Traveler’s Guide WILDLIFE WATCHINGTraveler’s IN PERU Guide WILDLIFE WATCHING IN PERU ORCHIDS Tumbes and Piura Amazonas and San Martín Huánuco and Tingo María Cordillera Blanca Chanchamayo Valley Machu Picchu Manu and Tambopata RECOMMENDATIONS LOCATION AND CLIMATE www.peru.org.pe [email protected] 2 Traveler’s Guide WILDLIFE WATCHINGTraveler’s IN PERU Guide WILDLIFE WATCHING IN PERU Peru, The Natural Destination Peru is, undoubtedly, one of the world’s top desti- For Peru, nature-tourism and eco-tourism repre- nations for nature-lovers. Blessed with the richest sent an opportunity to share its many surprises ocean in the world, largely unexplored Amazon for- and charm with the rest of the world. This guide ests and the highest tropical mountain range on provides descriptions of the main groups of species Pthe planet, the possibilities for the development of the country offers nature-lovers; trip recommen- bio-diversity in its territory are virtually unlim- dations; information on destinations; services and ited. -

Parallel Evolution in the Major Haemoglobin Genes of Eight Species of Andean Waterfowl
Molecular Ecology (2009) doi: 10.1111/j.1365-294X.2009.04352.x Parallel evolution in the major haemoglobin genes of eight species of Andean waterfowl K. G. M C CRACKEN,* C. P. BARGER,* M. BULGARELLA,* K. P. JOHNSON,† S. A. SONSTHAGEN,* J. TRUCCO,‡ T. H. VALQUI,§– R. E. WILSON,* K. WINKER* and M. D. SORENSON** *Institute of Arctic Biology, Department of Biology and Wildlife, and University of Alaska Museum, University of Alaska Fairbanks, Fairbanks, AK 99775, USA, †Illinois Natural History Survey, Champaign, IL 61820, USA, ‡Patagonia Outfitters, Perez 662, San Martin de los Andes, Neuque´n 8370, Argentina, §Centro de Ornitologı´a y Biodiversidad (CORBIDI), Sta. Rita 117, Urbana Huertos de San Antonio, Surco, Lima 33, Peru´, –Museum of Natural Science, Louisiana State University, Baton Rouge, LA 70803, USA, **Department of Biology, Boston University, Boston, MA 02215, USA Abstract Theory predicts that parallel evolution should be common when the number of beneficial mutations is limited by selective constraints on protein structure. However, confirmation is scarce in natural populations. Here we studied the major haemoglobin genes of eight Andean duck lineages and compared them to 115 other waterfowl species, including the bar-headed goose (Anser indicus) and Abyssinian blue-winged goose (Cyanochen cyanopterus), two additional species living at high altitude. One to five amino acid replacements were significantly overrepresented or derived in each highland population, and parallel substitutions were more common than in simulated sequences evolved under a neutral model. Two substitutions evolved in parallel in the aA subunit of two (Ala-a8) and five (Thr-a77) taxa, and five identical bA subunit substitutions were observed in two (Ser-b4, Glu-b94, Met-b133) or three (Ser-b13, Ser-b116) taxa. -

Notes on Some Argentine Anatids
NOTESON SOME ARGENTINEANATIDS' MILTON W. WELLER ROM mid-August, 1964, until late July, 1965, I was engaged in field work F in Argentina studying waterfowl. Although special emphasis was placed on the Black-headed Duck (Heteronetta atricapilla) , 28 species of anatids were observed in various parts of Argentina. Because so little is known of these species, some general observations are summarized and discussed in the hope that it will point out gaps in our knowledge and encourage additional work on this interesting group. Field work was financed by Grant GB-1067 from the National Science Foundation. Studies of museum specimens in the United States were financed by a Chapman Grant of the American Museum of Natural History, and aided materially in appraising the significance of plumage sequences in Neotropical ducks. AQUATIC HABITATS Although the distribution of Argentine birds was considered by Dabbene (1910) and by Olrog (19591, little comment has been made on the distribution of water types and their influence on waterfowl distribution. Some helpful botanical comments are given by Cabrera (1953) for the Buenos Aires region and, Wetmores’ (1926) observations on both botany and ornithology are excellent. Although time did not permit detailed botanical work during this study, some obvious differences in life-form of marsh vegetation were recorded in the areas visited (Fig. 1) in relation to the species composi- tion of waterbirds. The most extensive zone of freshwater and semipermanent marshes is found in an area roughly bordered by the cities of Venado Tuerto (Santa Fe), Buenos Aires, General Lavalle, Mar de1 Plats and Azul (Buenos Aires). -

Bolivia: Endemic Macaws & More!
BOLIVIA: ENDEMIC MACAWS & MORE! PART II: FOOTHILLS, CLOUDFORESTS & THE ALTIPLANO SEPTEMBER 28–OCTOBER 8, 2018 Male Versicolored Barbet – Photo Andrew Whittaker LEADERS: ANDREW WHITTAKER & JULIAN VIDOZ LIST COMPILED BY: ANDREW WHITTAKER VICTOR EMANUEL NATURE TOURS, INC. 2525 WALLINGWOOD DRIVE, SUITE 1003 AUSTIN, TEXAS 78746 WWW.VENTBIRD.COM Bolivia continued to exceed expectations on Part 2 of our tour! Steadily climbing up into the mighty ceiling of South America that is the Andes, we enjoyed exploring many more new, different, and exciting unspoiled bird-rich habitats, including magical Yungas cloudforest stretching as far as the eye could see; dry and humid Puna; towering snow-capped Andean peaks; vast stretches of Altiplano with its magical brackish lakes filled with immense numbers of glimmering flamingoes, and one of my favorite spots, the magnificent and famous Lake Titicaca (with its own flightless grebe). An overdose of stunning Andean scenery combined with marvelous shows of flowering plants enhanced our explorations of a never-ending array of different and exciting microhabitats for so many special and interesting Andean birds. We were rewarded with a fabulous trip record total of 341 bird species! Combining our two exciting Bolivia tours (Parts 1 and 2) gave us an all-time VENT record, an incredible grand total of 656 different bird species and 15 mammals! A wondrous mirage of glimmering pink hues of all three species of flamingos on the picturesque Bolivian Altiplano – Photo Andrew Whittaker Stunning Andes of Bolivia near Soroto on a clear day of our 2016 trip – Photo Andrew Whittaker Victor Emanuel Nature Tours 2 Bolivia Part 2, 2018 We began this second part of our Bolivian bird bonanza in the bustling city of Cochabamba, spending a fantastic afternoon birding the city’s rich lakeside in lovely late afternoon sun. -

Buceros Vol-9 No-3 Year-2004
Buceros Vol. 9, No. 3 (2004) A bibliography of the Anatidae of south Asia Aasheesh Pittie 8-2-545 Road No. 7, Banjara Hills, Hyderabad 500034, India. Email: [email protected] INTRODUCTION This bibliography has been extracted from my larger a distribution pattern of the Anatidae.Accuracy in bibliographic database (Pittie 2005) and covers the transcribing is a basic tenet of bibliography and though political boundaries of the following south Asian great care has been taken to ensure it, mistakes may countries: Afghanistan, Bangladesh, Bhutan, India, the have crept in and pertinent papers, notes, reports, books, Maldives, Myanmar (Burma), Nepal, Pakistan, and Sri etc., may have been inadvertently left out.This is the Lanka. Tibet is also covered. It comprises papers, popular more likely in that I have not seen all the entries listed articles, books, published and un-published reports, and below in the original, but have freely taken them second chapters, in which members of the avian family, Anatidae hand from the ‘References’ or ‘Further Reading’ sections (ducks, geese, swans), find mention. It covers a period of papers and books. of over two and a half centuries, from 1750 up to 2004. Authors have been arranged alphabetically and their Of the 49 genera and 158 species that comprise the work chronologically. Multi-author papers have been family Anatidae worldwide (Dickinson 2003), 19 genera listed under the name of the senior author(i.e., the first and 46 species are found in south Asia. Of these, the author, not the oldest). Separate entries have not been Pink-headed Duck Rhodonessa caryophyllacea is made for co-authors.