Cusumano-Et-Al-2017.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
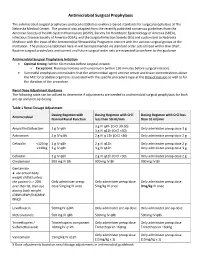
Antimicrobial Surgical Prophylaxis
Antimicrobial Surgical Prophylaxis The antimicrobial surgical prophylaxis protocol establishes evidence-based standards for surgical prophylaxis at The Nebraska Medical Center. The protocol was adapted from the recently published consensus guidelines from the American Society of Health-System Pharmacists (ASHP), Society for Healthcare Epidemiology of America (SHEA), Infectious Disease Society of America (IDSA), and the Surgical Infection Society (SIS) and customized to Nebraska Medicine with the input of the Antimicrobial Stewardship Program in concert with the various surgical groups at the institution. The protocol established here-in will be implemented via standard order sets utilized within One Chart. Routine surgical prophylaxis and current and future surgical order sets are expected to conform to this guidance. Antimicrobial Surgical Prophylaxis Initiation Optimal timing: Within 60 minutes before surgical incision o Exceptions: Fluoroquinolones and vancomycin (within 120 minutes before surgical incision) Successful prophylaxis necessitates that the antimicrobial agent achieve serum and tissue concentrations above the MIC for probable organisms associated with the specific procedure type at the time of incision as well as for the duration of the procedure. Renal Dose Adjustment Guidance The following table can be utilized to determine if adjustments are needed to antimicrobial surgical prophylaxis for both pre-op and post-op dosing. Table 1 Renal Dosage Adjustment Dosing Regimen with Dosing Regimen with CrCl Dosing Regimen with -

Antimicrobial Stewardship Guidance
Antimicrobial Stewardship Guidance Federal Bureau of Prisons Clinical Practice Guidelines March 2013 Clinical guidelines are made available to the public for informational purposes only. The Federal Bureau of Prisons (BOP) does not warrant these guidelines for any other purpose, and assumes no responsibility for any injury or damage resulting from the reliance thereof. Proper medical practice necessitates that all cases are evaluated on an individual basis and that treatment decisions are patient-specific. Consult the BOP Clinical Practice Guidelines Web page to determine the date of the most recent update to this document: http://www.bop.gov/news/medresources.jsp Federal Bureau of Prisons Antimicrobial Stewardship Guidance Clinical Practice Guidelines March 2013 Table of Contents 1. Purpose ............................................................................................................................................. 3 2. Introduction ...................................................................................................................................... 3 3. Antimicrobial Stewardship in the BOP............................................................................................ 4 4. General Guidance for Diagnosis and Identifying Infection ............................................................. 5 Diagnosis of Specific Infections ........................................................................................................ 6 Upper Respiratory Infections (not otherwise specified) .............................................................................. -

Susceptibility of Pseudomonas Cepacia Isolated from Children with Cystic Fibrosis1
003 1-3998/86/2011-1174$02.00/0 PEDIATRIC RESEARCH Vol. 20, No. 1 1, 1986 Copyright O 1986 International Pediatric Research Foundation, Inc. Printed in (I.S.A. Decreased Baseline P-Lactamase Production and Inducibility associated with Increased Piperacillin Susceptibility of Pseudomonas cepacia Isolated from Children with Cystic Fibrosis1 CLAUDIO CHIESA,~PAULINE H. LABROZZI, AND STEPHEN C. ARONOFF Department of Pediatrics [C.C., P.H.L., S.C.A.], Case- Western Reserve University School ofMedicine and the Division of Pediatric Infectious Diseases [S.C.A.], Rainbow Babies and Children's Hospital, Cleveland, Ohio ABSTRACT. The incidence of pulmonary infections in creased since 1978 and is now recovered from 20% of patients children with cystic fibrosis caused by Pseudomonas ce- in some centers (3, 4). pacia, an organism which may possess an inducible 8- Sputum isolates of P. cepacia from children with cystic fibrosis lactamase, has increased since 1978. Seven of 13 sputum are resistant to most P-lactam agents in vitro. In an in vitro study isolates of P. cepacia from children with cystic fibrosis comparing the susceptibilities of 62 consecutive sputum isolates, were classified as inducible by quantitative enzyme produc- concentrations of 64 pg/ml of aztreonam and piperacillin were tion following preincubation with 100, 200, or 400 pg/ml required to inhibit 79 and 87.1 % of the bacterial population, of cefoxitin. The recovery of inducible strains tended to be respectively; 90% of the isolates were inhibited by 8 pg/ml of associated with recent ceftazidime therapy. Susceptibility ceftazidime (5). In vitro, ceftazidime has proven more active to aztreonam, ceftazidime, and piperacillin alone or com- against P. -

Australian Public Assessment Refport for Ceftaroline Fosamil (Zinforo)
Australian Public Assessment Report for ceftaroline fosamil Proprietary Product Name: Zinforo Sponsor: AstraZeneca Pty Ltd May 2013 Therapeutic Goods Administration About the Therapeutic Goods Administration (TGA) • The Therapeutic Goods Administration (TGA) is part of the Australian Government Department of Health and Ageing, and is responsible for regulating medicines and medical devices. • The TGA administers the Therapeutic Goods Act 1989 (the Act), applying a risk management approach designed to ensure therapeutic goods supplied in Australia meet acceptable standards of quality, safety and efficacy (performance), when necessary. • The work of the TGA is based on applying scientific and clinical expertise to decision- making, to ensure that the benefits to consumers outweigh any risks associated with the use of medicines and medical devices. • The TGA relies on the public, healthcare professionals and industry to report problems with medicines or medical devices. TGA investigates reports received by it to determine any necessary regulatory action. • To report a problem with a medicine or medical device, please see the information on the TGA website <http://www.tga.gov.au>. About AusPARs • An Australian Public Assessment Record (AusPAR) provides information about the evaluation of a prescription medicine and the considerations that led the TGA to approve or not approve a prescription medicine submission. • AusPARs are prepared and published by the TGA. • An AusPAR is prepared for submissions that relate to new chemical entities, generic medicines, major variations, and extensions of indications. • An AusPAR is a static document, in that it will provide information that relates to a submission at a particular point in time. • A new AusPAR will be developed to reflect changes to indications and/or major variations to a prescription medicine subject to evaluation by the TGA. -

Comparative Ceftaroline Activity Tested Against Staphylococcus Aureus Associated with Acute Bacterial Skin and Skin Structure Infection from a Tertiary Care Center
RESEARCH PAPER Medical Science Volume : 5 | Issue : 4 | April 2015 | ISSN - 2249-555X Comparative Ceftaroline Activity Tested Against Staphylococcus Aureus Associated with Acute Bacterial Skin and Skin Structure Infection from A Tertiary Care Center KEYWORDS MRSA, Ceftaroline, vancomycin Dr. Umamageswari S. S. M. Dr. S. Habeeb Mohammed Dr. Shameem Banu Associate Professor- Microbiology Associate Professor-Surgery Professor and HOD- Microbiology Chettinad Hospital & Research Chettinad Hospital & Research Chettinad Hospital & Research Insitute Insitute Insitute Dr. Jayanthi S. Associate Professor- Microbiology Chettinad Hospital & Research Insitute ABSTRACT Methicillin resistant Staphylococcus aureus (MRSA), commonest cause of acute bacterial skin and skin structure infection (ABSSSI) has only limited treatment options like vancomycin, Linezolid and teicoplanin. Staphylococcus aureus with reduced susceptibilities to vancomycin is developing nowadays among nosocomial infec- tion. A newest cephalosporin – Ceftaroline has received FDA approval for the treatment of ABSSSI recently has unique activity against MRSA. The aim of the study is to know the prevalence of Staphylococcus aureus in ABSSSI patients and to evaluate the in vitro activity of Ceftaroline, in comparison with other drugs. Minimum Inhibitory Concentration for vancomycin and Ceftaroline were determined by E-test strips. Out of 235 Staphylococcus aureus isolated from ABSSSI patient 32% were MRSA. No vancomycin resistant strain isolated in our study. In vitro, Ceftaroline control the growth of MRSA very effectively at 2mg/ml itself. Ceftaroline is a most welcomed drug in the treatment of MRSA. INTRODUCTION: MATERIALS AND METHODS: Complicated skin and skin structure infection (CSSSIs), now This study was carried out in Chettinad health city and known by the new US FDA as Acute bacterial skin and skin Research Institute, a tertiary care center hospital (Chen- structure infections (ABSSSI) are the most common infec- nai) between April 2013 and May 2014. -

Penicillin Allergy Guidance Document
Penicillin Allergy Guidance Document Key Points Background Careful evaluation of antibiotic allergy and prior tolerance history is essential to providing optimal treatment The true incidence of penicillin hypersensitivity amongst patients in the United States is less than 1% Alterations in antibiotic prescribing due to reported penicillin allergy has been shown to result in higher costs, increased risk of antibiotic resistance, and worse patient outcomes Cross-reactivity between truly penicillin allergic patients and later generation cephalosporins and/or carbapenems is rare Evaluation of Penicillin Allergy Obtain a detailed history of allergic reaction Classify the type and severity of the reaction paying particular attention to any IgE-mediated reactions (e.g., anaphylaxis, hives, angioedema, etc.) (Table 1) Evaluate prior tolerance of beta-lactam antibiotics utilizing patient interview or the electronic medical record Recommendations for Challenging Penicillin Allergic Patients See Figure 1 Follow-Up Document tolerance or intolerance in the patient’s allergy history Consider referring to allergy clinic for skin testing Created July 2017 by Macey Wolfe, PharmD; John Schoen, PharmD, BCPS; Scott Bergman, PharmD, BCPS; Sara May, MD; and Trevor Van Schooneveld, MD, FACP Disclaimer: This resource is intended for non-commercial educational and quality improvement purposes. Outside entities may utilize for these purposes, but must acknowledge the source. The guidance is intended to assist practitioners in managing a clinical situation but is not mandatory. The interprofessional group of authors have made considerable efforts to ensure the information upon which they are based is accurate and up to date. Any treatments have some inherent risk. Recommendations are meant to improve quality of patient care yet should not replace clinical judgment. -

TML\MSH Microbiology Department Policy & Procedure Manual Policy
TML\MSH Microbiology Department Policy # MI\ANTI\04\03\v03 Page 1 of 5 Policy & Procedure Manual Section: Antimicrobial Susceptibility Testing Subject Title: Appendix III - Double Disk Manual Test for ESBL Issued by: LABORATORY MANAGER Original Date: January 10, 2000 Approved by: Laboratory Director Revision Date: November 21, 2005 APPENDIX III - DOUBLE DISK TEST FOR ESBL I. Introduction Class A or Bush Group 1 extended spectrum beta-lactamases (ESBLs) are inhibited by clavulanic acid. This may be detected by testing the suspected organism to a 3rd generation cephalosporin alone and in combination with clavulanic acid. If the combination results in an expanded zone of inhibition compared to that of the 3rd generation cephalosporin alone, it is indicative of the presence of an ESBL. II. Materials Mueller-Hinton (MH) agar (150) mm 20/10 mg amoxicillin-clavulanate disc 30 mg ceftazidime disc 30 mg ceftriaxone or cefotaxime disc 30 mg aztreonam disc 10 mg cefpodoxime disc (optional) 30 mg cefoxitin disc Quality control strain: E. coli ATCC 51446 III. Procedure 1. Prepare a bacterial suspension of the organism to be tested that has a turbidity equivalent to that of a 0.5 McFarland standard. 2. Inoculate a Mueller-Hinton agar plate with this suspension in accordance with NCCLS M100-S10 (M2) guidelines for disc diffusion testing. 3. Place the amoxicillin-clavulanic acid disc towards the centre of the plate. 4. Carefully measure 15 mm out from the edge of that disc at 90o angles marking the plate. 5. Place a ceftazidime disk on the plate so that its inner edge is 15 mm (the mark) from the amoxicillin-clavulanic acid disc (See Figure 1 KB-ESBL Template). -

Ampicillin/Sulbactam Vs. Cefoxitin for the Treatment of Pelvic Inflammatory Disease
Infectious Diseases in Obstetrics and Gynecology 5:319–325 (1997) © 1998 Wiley-Liss, Inc. Ampicillin/Sulbactam Vs. Cefoxitin for the Treatment of Pelvic Inflammatory Disease Joseph G. Jemsek* and Frank Harrison Department of Obstetrics and Gynecology, Charlotte Memorial Hospital, Charlotte, NC ABSTRACT Objective: The safety and efficacy of ampicillin plus sulbactam were compared with those of ce- foxitin in the treatment of women with pelvic inflammatory disease (PID). Methods: This single-site, randomized, prospective, third-party-blinded, comparative, parallel- treatment study enrolled 93 women with a diagnosis of PID. Patients were treated with either ampicillin/sulbactam (2 g/1 g, administered intravenously [IV], every 6 h) or cefoxitin (2 g, admin- istered IV, every 6 h) for a minimum of 12 doses. Patients with cultures positive for Chlamydia trachomatis also received concurrent oral or IV doxycycline (100 mg twice daily). Patients with cultures negative for C. trachomatis received prophylactic oral doxycycline (100 mg twice daily) for 10–14 days after treatment with either ampicillin/sulbactam or cefoxitin was completed. Results: Ninety-three patients were entered in the study: 47 in the ampicillin/sulbactam arm and 46 in the cefoxitin arm. All 93 patients were evaluable for safety; 61 (66%) were evaluable for efficacy. Demographic characteristics were similar for the groups. Of the 27 evaluable ampicillin/ sulbactam-treated patients, 67% experienced clinical cure, 30% improved, and 4% failed treatment. Respective values for the 34 cefoxitin-treated patients were 68%, 24%, and 9% (P = 0.67). Patho- gens were eradicated in 70% of the women given ampicillin/sulbactam vs. 56% of those who re- ceived cefoxitin (P = 0.64). -

Management of Penicillin and Beta-Lactam Allergy
Management of Penicillin and Beta-Lactam Allergy (NB Provincial Health Authorities Anti-Infective Stewardship Committee, September 2017) Key Points • Beta-lactams are generally safe; allergic and adverse drug reactions are over diagnosed and over reported • Nonpruritic, nonurticarial rashes occur in up to 10% of patients receiving penicillins. These rashes are usually not allergic and are not a contraindication to the use of a different beta-lactam • The frequently cited risk of 8 to 10% cross-reactivity between penicillins and cephalosporins is an overestimate based on studies from the 1970’s that are now considered flawed • Expect new intolerances (i.e. any allergy or adverse reaction reported in a drug allergy field) to be reported after 0.5 to 4% of all antimicrobial courses depending on the gender and specific antimicrobial. Expect a higher incidence of new intolerances in patients with three or more prior medication intolerances1 • For type-1 immediate hypersensitivity reactions (IgE-mediated), cross-reactivity among penicillins (table 1) is expected due to similar core structure and/or major/minor antigenic determinants, use not recommended without desensitization • For type-1 immediate hypersensitivity reactions, cross-reactivity between penicillins (table 1) and cephalosporins is due to similarities in the side chains; risk of cross-reactivity will only be significant between penicillins and cephalosporins with similar side chains • Only type-1 immediate hypersensitivity to a penicillin manifesting as anaphylaxis, bronchospasm, -

Cephalosporins Can Be Prescribed Safely for Penicillin-Allergic Patients ▲
JFP_0206_AE_Pichichero.Final 1/23/06 1:26 PM Page 106 APPLIED EVIDENCE New research findings that are changing clinical practice Michael E. Pichichero, MD University of Rochester Cephalosporins can be Medical Center, Rochester, NY prescribed safely for penicillin-allergic patients Practice recommendations an allergic reaction to cephalosporins, ■ The widely quoted cross-allergy risk compared with the incidence of a primary of 10% between penicillin and (and unrelated) cephalosporin allergy. cephalosporins is a myth (A). Most people produce IgG and IgM antibodies in response to exposure to ■ Cephalothin, cephalexin, cefadroxil, penicillin1 that may cross-react with and cefazolin confer an increased risk cephalosporin antigens.2 The presence of of allergic reaction among patients these antibodies does not predict allergic, with penicillin allergy (B). IgE cross-sensitivity to a cephalosporin. ■ Cefprozil, cefuroxime, cefpodoxime, Even penicillin skin testing is generally not ceftazidime, and ceftriaxone do not predictive of cephalosporin allergy.3 increase risk of an allergic reaction (B). Reliably predicting cross-reactivity ndoubtedly you have patients who A comprehensive review of the evidence say they are allergic to penicillin shows that the attributable risk of a cross- U but have difficulty recalling details reactive allergic reaction varies and is of the reactions they experienced. To be strongest when the chemical side chain of safe, we often label these patients as peni- the specific cephalosporin is similar to that cillin-allergic without further questioning of penicillin or amoxicillin. and withhold not only penicillins but Administration of cephalothin, cepha- cephalosporins due to concerns about lexin, cefadroxil, and cefazolin in penicillin- potential cross-reactivity and resultant IgE- allergic patients is associated with a mediated, type I reactions. -

Mycobacterium Abscessus Pulmonary Disease: Individual Patient Data Meta-Analysis
ORIGINAL ARTICLE RESPIRATORY INFECTIONS Mycobacterium abscessus pulmonary disease: individual patient data meta-analysis Nakwon Kwak1, Margareth Pretti Dalcolmo2, Charles L. Daley3, Geoffrey Eather4, Regina Gayoso2, Naoki Hasegawa5, Byung Woo Jhun 6, Won-Jung Koh 6, Ho Namkoong7, Jimyung Park1, Rachel Thomson8, Jakko van Ingen 9, Sanne M.H. Zweijpfenning10 and Jae-Joon Yim1 @ERSpublications For Mycobacterium abscessus pulmonary disease in general, imipenem use is associated with improved outcome. For M. abscessus subsp. abscessus, the use of either azithromycin, amikacin or imipenem increases the likelihood of treatment success. http://ow.ly/w24n30nSakf Cite this article as: Kwak N, Dalcolmo MP, Daley CL, et al. Mycobacterium abscessus pulmonary disease: individual patient data meta-analysis. Eur Respir J 2019; 54: 1801991 [https://doi.org/10.1183/ 13993003.01991-2018]. ABSTRACT Treatment of Mycobacterium abscessus pulmonary disease (MAB-PD), caused by M. abscessus subsp. abscessus, M. abscessus subsp. massiliense or M. abscessus subsp. bolletii, is challenging. We conducted an individual patient data meta-analysis based on studies reporting treatment outcomes for MAB-PD to clarify treatment outcomes for MAB-PD and the impact of each drug on treatment outcomes. Treatment success was defined as culture conversion for ⩾12 months while on treatment or sustained culture conversion without relapse until the end of treatment. Among 14 eligible studies, datasets from eight studies were provided and a total of 303 patients with MAB-PD were included in the analysis. The treatment success rate across all patients with MAB-PD was 45.6%. The specific treatment success rates were 33.0% for M. abscessus subsp. abscessus and 56.7% for M. -

Mycobacterium Avium Complex Genitourinary Infections: Case Report and Literature Review
Case Report Mycobacterium Avium Complex Genitourinary Infections: Case Report and Literature Review Sanu Rajendraprasad 1, Christopher Destache 2 and David Quimby 1,* 1 School of Medicine, Creighton University, Omaha, NE 68124, USA; [email protected] 2 College of Pharmacy, Creighton University, Omaha, NE 68124, USA; [email protected] * Correspondence: [email protected] Abstract: Nontuberculous mycobacterial (NTM) genitourinary (GU) infections are relatively rare, and there is frequently a delay in diagnosis. Mycobacterium avium-intracellulare complex (MAC) cases seem to be less frequent than other NTM as a cause of these infections. In addition, there are no set treatment guidelines for these organisms in the GU tract. Given the limitations of data this review summarizes a case presentation of this infection and the literature available on the topic. Many different antimicrobial regimens and durations have been used in the published literature. While the infrequency of these infections suggests that there will not be randomized controlled trials to determine optimal therapy, our case suggests that a brief course of amikacin may play a useful role in those who cannot tolerate other antibiotics. Keywords: nontuberculous mycobacteria; mycobacterium avium-intracellulare complex; urinary tract infections; genitourinary infections Citation: Rajendraprasad, S.; Destache, C.; Quimby, D. 1. Introduction Mycobacterium Avium Complex In recent decades, the incidence and prevalence of nontuberculous mycobacteria Genitourinary Infections: Case (NTM) causing extrapulmonary infections have greatly increased, becoming a major world- Report and Literature Review. Infect. wide public health problem [1,2]. Among numerous NTM species, the Mycobacterium avium Dis. Rep. 2021, 13, 454–464. complex (MAC) is the most common cause of infection in humans.