Wöhler Synthesis of Urea
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Oh Ho Oh Oh Oh Oh O Ho Ch3o N N H O Ho Oh Oh Oh Oh O Ho Ch3o N N
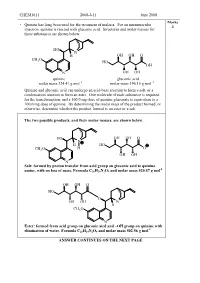
CHEM1611 2008-J-11 June 2008 Marks • Quinine has long been used for the treatment of malaria. For an intramuscular 4 injection, quinine is reacted with gluconic acid. Structures and molar masses for these substances are shown below. HO N H OH OH O CH O 3 HO OH N OH OH quinine gluconic acid molar mass 324.41 g mol –1 molar mass 196.16 g mol –1 Quinine and gluconic acid can undergo an acid-base reaction to form a salt, or a condensation reaction to form an ester. One molecule of each substance is required for the transformation, and a 160.0 mg dose of quinine gluconate is equivalent to a 100.0 mg dose of quinine. By determining the molar mass of the product formed, or otherwise, determine whether the product formed is an ester or a salt. The two possible products, and their molar masses, are shown below. HO OH OH O N H HO CH3O H O OH OH N Salt: formed by proton transfer from acid group on gluconic acid to quinine -1 amine, with no loss of mass. Formula C 26 H26 N2O9 and molar mass 520.57 g mol OH OH O HO O OH OH N H CH3O N Ester: formed from acid group on gluconic aicd and –OH group on quinine with -1 elimination of water. Formula C 26 H24 N2O8 and molar mass 502.56 g mol ANSWER CONTINUES ON THE NEXT PAGE CHEM1611 2008-J-11 June 2008 100.0 mg of quinine corresponds to: ̡̭̳̳ ̊̉̉.̉Ɛ̊̉ ̌ ̧ number of moles = Ɣ = 3.083 × 10 -4 mol ̡̭̯̬̲ ̡̭̳̳ ̌̋̍.̍̊ ̧ ̭̯̬ ̊ 160.0 mg of the salt product corresponds to: ̡̭̳̳ ̊̏̉.̉Ɛ̊̉ ̌ ̧ number of moles = Ɣ = 3.074 × 10 -4 mol ̡̭̯̬̲ ̡̭̳̳ ̎̋̉.̎̐ ̧ ̭̯̬ ̊ 160.0 mg of the ester product corresponds to: ̡̭̳̳ ̊̏̉.̉Ɛ̊̉ ̌ ̧ number of moles = Ɣ = 3.184 × 10 -4 mol ̡̭̯̬̲ ̡̭̳̳ ̎̉̋.̎̏ ̧ ̭̯̬ ̊ As the dosages are the same, it must be the salt which is being administered. -

Retention Indices for Frequently Reported Compounds of Plant Essential Oils
Retention Indices for Frequently Reported Compounds of Plant Essential Oils V. I. Babushok,a) P. J. Linstrom, and I. G. Zenkevichb) National Institute of Standards and Technology, Gaithersburg, Maryland 20899, USA (Received 1 August 2011; accepted 27 September 2011; published online 29 November 2011) Gas chromatographic retention indices were evaluated for 505 frequently reported plant essential oil components using a large retention index database. Retention data are presented for three types of commonly used stationary phases: dimethyl silicone (nonpolar), dimethyl sili- cone with 5% phenyl groups (slightly polar), and polyethylene glycol (polar) stationary phases. The evaluations are based on the treatment of multiple measurements with the number of data records ranging from about 5 to 800 per compound. Data analysis was limited to temperature programmed conditions. The data reported include the average and median values of retention index with standard deviations and confidence intervals. VC 2011 by the U.S. Secretary of Commerce on behalf of the United States. All rights reserved. [doi:10.1063/1.3653552] Key words: essential oils; gas chromatography; Kova´ts indices; linear indices; retention indices; identification; flavor; olfaction. CONTENTS 1. Introduction The practical applications of plant essential oils are very 1. Introduction................................ 1 diverse. They are used for the production of food, drugs, per- fumes, aromatherapy, and many other applications.1–4 The 2. Retention Indices ........................... 2 need for identification of essential oil components ranges 3. Retention Data Presentation and Discussion . 2 from product quality control to basic research. The identifi- 4. Summary.................................. 45 cation of unknown compounds remains a complex problem, in spite of great progress made in analytical techniques over 5. -

Poxviruses: Smallpox Vaccine, Its Complications and Chemotherapy
Virus Adaptation and Treatment Dovepress open access to scientific and medical research Open Access Full Text Article R E V IEW Poxviruses: smallpox vaccine, its complications and chemotherapy Mimi Remichkova Abstract: The threat of bioterrorism in the recent years has once again posed to mankind the unresolved problems of contagious diseases, well forgotten in the past. Smallpox (variola) is Department of Pathogenic Bacteria, The Stephan Angeloff Institute among the most dangerous and highly contagious viral infections affecting humans. The last of Microbiology, Bulgarian Academy natural case in Somalia marked the end of a successful World Health Organization campaign of Sciences, Sofia, Bulgaria for smallpox eradication by vaccination on worldwide scale. Smallpox virus still exists today in some laboratories, specially designated for that purpose. The contemporary response in the treatment of the post-vaccine complications, which would occur upon enforcing new programs for mass-scale smallpox immunization, includes application of effective chemotherapeutics and their combinations. The goals are to provide the highest possible level of protection and safety of For personal use only. the population in case of eventual terrorist attack. This review describes the characteristic features of the poxviruses, smallpox vaccination, its adverse reactions, and poxvirus chemotherapy. Keywords: poxvirus, smallpox vaccine, post vaccine complications, inhibitors Characteristics of poxviruses Smallpox (variola) infection is caused by the smallpox virus. This virus belongs to the genus of Orthopoxvirus included in the Poxviridae family. Poxviruses are one of the largest and most complexly structured viruses, known so far. The genome of poxviruses consists of a linear two-chained DNA and its replication takes place in the cytoplasm of the infected cell. -

126.-In-Vitro-Prototyping-Of-Limonene-Biosynthesis.Pdf
Metabolic Engineering 61 (2020) 251–260 Contents lists available at ScienceDirect Metabolic Engineering journal homepage: www.elsevier.com/locate/meteng In vitro prototyping of limonene biosynthesis using cell-free protein synthesis T ∗ Quentin M. Dudley1, Ashty S. Karim, Connor J. Nash, Michael C. Jewett Department of Chemical and Biological Engineering and Center for Synthetic Biology, Northwestern University, Evanston, IL, 60208, USA ARTICLE INFO ABSTRACT Keywords: Metabolic engineering of microorganisms to produce sustainable chemicals has emerged as an important part of Cell-free metabolic engineering the global bioeconomy. Unfortunately, efforts to design and engineer microbial cell factories are challenging Limonene because design-build-test cycles, iterations of re-engineering organisms to test and optimize new sets of enzymes, iPROBE are slow. To alleviate this challenge, we demonstrate a cell-free approach termed in vitro Prototyping and Rapid Cell-free metabolic pathway prototyping Optimization of Biosynthetic Enzymes (or iPROBE). In iPROBE, a large number of pathway combinations can be Cell-free protein synthesis rapidly built and optimized. The key idea is to use cell-free protein synthesis (CFPS) to manufacture pathway Synthetic biology enzymes in separate reactions that are then mixed to modularly assemble multiple, distinct biosynthetic path- ways. As a model, we apply our approach to the 9-step heterologous enzyme pathway to limonene in extracts from Escherichia coli. In iterative cycles of design, we studied the impact of 54 enzyme homologs, multiple enzyme levels, and cofactor concentrations on pathway performance. In total, we screened over 150 unique sets of enzymes in 580 unique pathway conditions to increase limonene production in 24 h from 0.2 to 4.5 mM (23–610 mg/L). -

Download File
Combined Biosynthetic and Synthetic Production of Valuable Molecules: A Hybrid Approach to Vitamin E and Novel Ambroxan Derivatives Bertrand T. Adanve Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2015 © 2015 Bertrand T. Adanve All Rights Reserved ABSTRACT Combined Biosynthetic and Synthetic Production of Valuable Molecules: A Hybrid Approach to Vitamin E and Novel Ambroxan Derivatives Bertrand T. Adanve Chapter 1. Introduction Synthetic chemistry has played a pivotal role in the evolution of modern life. More recently, the emerging field of synthetic biology holds the promise to bring about a paradigm shift with designer microbes to renewably synthesize complex molecules in a fraction of the time and cost. Still, given nature’s virtuosity at stitching a staggering palette of carbon frameworks with ease and synthetic chemistry’s superior parsing powers to access a greater number of unnatural end products, a hybrid approach that leverages the respective strengths of the two fields could prove advantageous for the efficient production of valuable natural molecules and their analogs. To demonstrate this approach, from biosynthetic Z,E-farnesol, we produced a library of novel analogs of the commercially important amber fragrance Ambrox®, including the first synthetic patchouli scent. Likewise, we produced the valuable tocotrienols from yeast-produced geranylgeraniol in a single step, the first such process of its kind. The novel acid catalyst system that allowed for this unique regioselective cyclization holds promise as an asymmetric proton transfer tool and could open the door to facile asymmetric synthesis of vitamin E and other molecules. -

Update of the LIPID MAPS Comprehensive Classification System for Lipids1
Update of the LIPID MAPS comprehensive classification system for lipids1 † Eoin Fahy,* Shankar Subramaniam, Robert C. Murphy,§ Masahiro Nishijima,** †† ††† Christian R. H. Raetz, Takao Shimizu,§§ Friedrich Spener,*** Gerrit van Meer, Michael J. O. Wakelam,§§§ and Edward A. Dennis2,**** † San Diego Supercomputer Center,* Department of Bioengineering, and Department of Chemistry and Biochemistry and Department of Pharmacology of the School of Medicine,**** University of California, San Diego, La Jolla, CA 92093-0505; Department of Pharmacology,§ University of Colorado Denver, Aurora, CO 80045-0598; National Institute of Health Sciences,** Setagaya-ku, Tokyo 158-8501, Japan; Department †† of Biochemistry, Duke University Medical Center, Durham, NC 27710; Department of Biochemistry and Molecular Biology,§§ Faculty of Medicine, University of Tokyo, Tokyo 113-0033, Japan; Department of Molecular Biosciences,*** University of Graz, 8010 Graz, Austria; Bijvoet Center and Institute of ††† Biomembranes, Utrecht University, 3584 CH Utrecht, The Netherlands; and The Babraham Institute,§§§ Babraham Research Campus, Cambridge, CB22 3AT, United Kingdom Abstract In 2005, the International Lipid Classification Nomenclature Committee (ILCNC) developed a “Com- and Nomenclature Committee under the sponsorship of prehensive Classification System for Lipids” that was pub- the LIPID MAPS Consortium developed and established a lished in 2005 (1). For the purpose of classification, we “ ” Comprehensive Classification System for Lipids based define lipids as hydrophobic or amphipathic small mole- on well-defined chemical and biochemical principles and cules that may originate entirely or in part by carbanion- using an ontology that is extensible, flexible, and scalable. This classification system, which is compatible with contem- based condensations of thioesters (fatty acyls, glycerolipids, porary databasing and informatics needs, has now been ac- glycerophospholipids, sphingolipids, saccharolipids, and cepted internationally and widely adopted. -

Menthol Smokers: Metabolomic Profiling and Smoking Behavior
Published OnlineFirst September 14, 2016; DOI: 10.1158/1055-9965.EPI-16-0124 Research Article Cancer Epidemiology, Biomarkers Menthol Smokers: Metabolomic Profiling & Prevention and Smoking Behavior Ping-Ching Hsu1,2, Renny S. Lan1, Theodore M. Brasky1, Catalin Marian1,3, Amrita K. Cheema4, Habtom W. Ressom4, Christopher A. Loffredo4, Wallace B. Pickworth5, and Peter G. Shields1 Abstract Background: The use of menthol in cigarettes and mar- tial least squares-discriminant analysis (PLS-DA) was carried keting is under consideration for regulation by the FDA. out for the classification of metabolomics profiles. However, the effects of menthol on smoking behavior and Results: MG boost was positively correlated with CO boost, carcinogen exposure have been inconclusive. We previously nicotine boost, average puff volume, puff duration, and total reported metabolomic profiling for cigarette smokers, and smoke exposure. Classification using PLS-DA, MG was the top novelly identified a menthol-glucuronide (MG) as the most metabolite discriminating metabolome of menthol versus non- significant metabolite directly related to smoking. Here, MG menthol smokers. Among menthol smokers, 42 metabolites were is studied in relation to smoking behavior and metabolomic significantly correlated with MG boost, which linked to cellular profiles. functions, such as of cell death, survival, and movement. Methods: This is a cross-sectional study of 105 smokers who Conclusions: Plasma MG boost is a new smoking behavior smoked two cigarettes in the laboratory one hour apart. Blood biomarker that may provide novel information over self-reported nicotine, MG, and exhaled carbon monoxide (CO) boosts were use of menthol cigarettes by integrating different smoking mea- determined (the difference before and after smoking). -

Blister Beetles in Alfalfa Circular 536 Revised by Jane Breen Pierce1
Blister Beetles in Alfalfa Circular 536 Revised by Jane Breen Pierce1 Cooperative Extension Service • College of Agricultural, Consumer and Environmental Sciences This publication provides information on the veterinary Table 1. Estimated Number of Beetles for a Lethal and agronomic importance, distinguishing features, (1 mg/kg) Dose of Cantharidin biology, distribution, and control of blister beetles. Beetle Horse Weight (lb) Recommendations for the purchase and use of alfalfa Cantharidin hay by horse owners and other livestock owners are Content (mg) 275 550 1,000 also provided. 1 125 250 455 2 63 125 244 3 41 83 161 VETERINARY SIGNIFICANCE OF BLISTER BEETLES 4 31 63 122 The common name for blister beetles comes from the 5 25 50 97 irritating reaction the beetle’s body fluids cause on ani- Adapted from Campinera et al. (1985) mal skin or delicate membranes. These fluids contain cantharidin, a potent blistering agent that is present in varying amounts in most blister beetle species. Fluids are blistering of the mouth, esophagus, stomach, and blad- released when the beetle is crushed or handled roughly. der. Death can occur 24 hours after a heavy dose. Cantharidin is a stable chemical and a long-term health Laboratory studies have been conducted to determine threat to nearly all livestock (particularly horses) that are the amount of cantharidin contained in various species fed contaminated hay. Storing infested hay does not sig- of blister beetles. Reports on beetles in several genera nificantly reduce the amount of cantharidin in the hay. indicate cantharidin content varying from 1 to 11.3% Research reports indicate cantharidin toxosis can be of their dry weight. -

BLISTER BEETLES in ALFALFA L.Ee Townsend, Extension Entomologist
U N I V E R S I T Y O F K E N T U C K Y COLLEGE OF AGRICULTURE DEPARTMENT OF ENTOMOLOGY ENTFACT-102 BLISTER BEETLES IN ALFALFA L.ee Townsend, Extension Entomologist Several of the common members of this group of Female blister beetles lay clusters of eggs in the soil in beetles contain a chemical that often causes blisters late summer. The small, active larvae that hatch from when applied to the skin; thus the name blister beetles. these eggs crawl over the soil surface entering cracks The substance can be toxic to animals that eat a in search for grasshopper egg pods which are deposited sufficient amount. An understanding of the insects and in the soil. After finding the eggmass, blister beetle their life cycles allows sound management practices to larvae become immobile and spend the rest of their minimize the chances of trapping beetles in hay. It also developmental time as legless grubs. The following gives horse and livestock owners information to summer they transform into the pupal stage and soon consider when making hay purchases. One major emerge in the adult stage. This is why blister beetle factor that increases potential for blister beetle numbers increase dramatically following high problems is crimping hay. This crushes the beetles and grasshopper populations. leaves them in the hay where they can be eaten by animals. The second factor is a large increase in Blister Beetle Toxicity grasshopper numbers. The larval stages of these blister Cantharidin is the poisonous substance in blister beetles develop on grasshopper egg pods in the soil. -

An Artifact in a Synthetic Pine Oil
RESEARCH NOTE J. Ess. Oil Res., 3, 41-42 (Jan/Feb 1991) An Artifact in a Synthetic Pine Oil Duane F. Zinkel USDA Forest Service, Forest Products Laboratory* One Gifford Pinchot Drive Madison, WI 53705-2398 ABSTRACT: The isopropyl ether of a-terpineol was identified as an artifact in the synthetic pine oil produced when isopropyl alcohol was used as the emulsifier. KEY WORD INDEX: Synthetic pine oil, a-terpineol isopropyl ether, terpinen-4-ol isopropyl ether, turpentine. INTRODUCTION: The manufacture of synthetic pine oil is the primary use for turpentine. The synthesis involves the acid-catalyzed hydration of a-pinene at the in terface ofan emulsion of pinene/mineral acid (1). Various emulsifiers have been used, one of which is isopropyl alcohol. Our gas chromatographic examination of a commercial distilled pine oil, produced using the isopropyl alcohol emulsifier, revealed the presence of 4-5% of a higher boiling component product not present originally in the turpentine. EXPERIMENTAL: NMR spectra were obtained at 310 K with a Bruker WM250 (250 MHz proton and 62.9 MHz carbon) FT spectrometer controlled by an Aspect 2000A minicomputer; DEPT spectra were obtained with a standard Bruker program. Gas chromatography was done with a Hewlett Packard 5880 gas chromatograph (FID) and fused-silica columns: a DB-1 (a methyl silicone) column from J & W Scientific (Folsom, CA), 15m x 0.25mmi.d. witha 0.1-µmfilmoperatedat60°Cand a Carbowaxcolumn,30m x 0.25mm with a 0.25-µm film temperature programmed from 60°C to 225°C at 8°C/min. isopropyl etherwas isolated by liquid chromatography. -

(19) United States (12) Patent Application Publication (10) Pub
US 20060081822A1 (19) United States (12) Patent Application Publication (10) Pub. N0.: US 2006/0081822 A1 Koetzle (43) Pub. Date: Apr. 20, 2006 (54) METHOD TO INCREASE FLASH POINTS OF (52) US. Cl. ............................................................ .. 252/601 FLAMMABLE SOLVENTS (76) Inventor: A. Richard Koetzle, Rochester, NY (57) ABSTRACT (Us) Correspondence AddreSSI The present invention relates to a method to decrease the A- RICHARD KOETZLE ?ammability of normally ?ammable alcohols and solvents. 134 STONECLIFF DRIVE The additive is Alpha Terpineol, Which Will increase the ROCHESTER’ NY 14616 (Us) ?ash point of ?ammable alcohols or solvents, by blending the Terpineol into the ?ammable solvent or alcohol. Solvents (21) Appl' NO" 10/968’441 such as acetone, methanol, ethylacetate, ethanol and Xylene, (22) Filed, Oct 20 2004 to name a feW, increases ?ash points by 50° C. to 60° C., by i ’ addition of 12-14% terpineol. The said solvent can then be Publication Classi?cation blended With other organic solvents to produce performance solvents, such as paint strippers With ?ash points greater (51) Int. Cl. than 1400 F. and meet Federal and state Volatile Organic C 09K 21/00 (2006.01) Compound regulations. US 2006/0081822 A1 Apr. 20, 2006 METHOD TO INCREASE FLASH POINTS OF [0008] The organic solvent or combination of solvents can FLAMMABLE SOLVENTS comprise up to 99 Weight percent of the composition in total, and may be the combination of tWo or more different types BACKGROUND OF THE INVENTION of organic solvents. A typical combination may comprise; [0001] Many industrial processing cleaning compositions [0009] 1.0 to 99 Weight percent organic solvent. -

Promising Strain of Acinetobacter from Soil for Utilization of Gluconic Acid Production Topraktaki Gelecek Vaadeden Acinetobacte
S. Dinç et al. / Hacettepe J. Biol. & Chem., 2017, 45 (4), 603-607 Promising Strain of Acinetobacter from Soil for Utilization of Gluconic Acid Production Topraktaki Gelecek Vaadeden Acinetobacter Suşunun Glukonik Asit Üretiminde Kullanımı Research Article Saliha Dinç 1,2*, Meryem Kara2,3, Mehmet Öğüt3, Fatih Er1, Hacer Çiçekçi2 1Selcuk University Cumra School of Applied Sciences, Konya-Turkey. 2Selcuk Uni. Advanced Technology Research and Application Center, Konya-Turkey. 3Selcuk University Cumra Vocational High School, Konya-Turkey. ABSTRACT luconic acid, a food additive, is used in many foods to control acidity or binds metals such as calcium, Giron. Acinetobacter sp. WR326, newly isolated from soil possesses high phosphate solubilizing activity and do not require pyrroloquinoline quinone (PQQ) for glucose dehydrogenase (GDH) activity as cofactor In this study the gluconic acid production potential of this bacterium was investigated. Firstly, Acinetobacter sp. WR326 was incubated in tricalcium phosphate medium (TCP) with varying glucose concentrations (100, 250, 500 mM), at a temperature of 30°C for 5 days (120 hours). The highest gluconic acid yield (59%) was found at a glucose concentration of 100 mM. Then three different levels of gluconic acid addition to the medium (50, 100, 200 mM) were tested. When Acinetobacter sp. WR326 strain was cultivated with a 100 mM glucose and 100 mM gluconic acid the yield increased to 95.27%. In any trials 2 -keto D-gluconic acid, causes problems in processing and purification of the gluconic acid, was not detected in the medium. As a conclusion, Acinetobac- ter sp. WR326 may be considered novel potential bacterial strain for gluconic acid production.