10.13 Planetary Atmospheres
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Introduction to Astronomy from Darkness to Blazing Glory
Introduction to Astronomy From Darkness to Blazing Glory Published by JAS Educational Publications Copyright Pending 2010 JAS Educational Publications All rights reserved. Including the right of reproduction in whole or in part in any form. Second Edition Author: Jeffrey Wright Scott Photographs and Diagrams: Credit NASA, Jet Propulsion Laboratory, USGS, NOAA, Aames Research Center JAS Educational Publications 2601 Oakdale Road, H2 P.O. Box 197 Modesto California 95355 1-888-586-6252 Website: http://.Introastro.com Printing by Minuteman Press, Berkley, California ISBN 978-0-9827200-0-4 1 Introduction to Astronomy From Darkness to Blazing Glory The moon Titan is in the forefront with the moon Tethys behind it. These are two of many of Saturn’s moons Credit: Cassini Imaging Team, ISS, JPL, ESA, NASA 2 Introduction to Astronomy Contents in Brief Chapter 1: Astronomy Basics: Pages 1 – 6 Workbook Pages 1 - 2 Chapter 2: Time: Pages 7 - 10 Workbook Pages 3 - 4 Chapter 3: Solar System Overview: Pages 11 - 14 Workbook Pages 5 - 8 Chapter 4: Our Sun: Pages 15 - 20 Workbook Pages 9 - 16 Chapter 5: The Terrestrial Planets: Page 21 - 39 Workbook Pages 17 - 36 Mercury: Pages 22 - 23 Venus: Pages 24 - 25 Earth: Pages 25 - 34 Mars: Pages 34 - 39 Chapter 6: Outer, Dwarf and Exoplanets Pages: 41-54 Workbook Pages 37 - 48 Jupiter: Pages 41 - 42 Saturn: Pages 42 - 44 Uranus: Pages 44 - 45 Neptune: Pages 45 - 46 Dwarf Planets, Plutoids and Exoplanets: Pages 47 -54 3 Chapter 7: The Moons: Pages: 55 - 66 Workbook Pages 49 - 56 Chapter 8: Rocks and Ice: -

Glossary Glossary
Glossary Glossary Albedo A measure of an object’s reflectivity. A pure white reflecting surface has an albedo of 1.0 (100%). A pitch-black, nonreflecting surface has an albedo of 0.0. The Moon is a fairly dark object with a combined albedo of 0.07 (reflecting 7% of the sunlight that falls upon it). The albedo range of the lunar maria is between 0.05 and 0.08. The brighter highlands have an albedo range from 0.09 to 0.15. Anorthosite Rocks rich in the mineral feldspar, making up much of the Moon’s bright highland regions. Aperture The diameter of a telescope’s objective lens or primary mirror. Apogee The point in the Moon’s orbit where it is furthest from the Earth. At apogee, the Moon can reach a maximum distance of 406,700 km from the Earth. Apollo The manned lunar program of the United States. Between July 1969 and December 1972, six Apollo missions landed on the Moon, allowing a total of 12 astronauts to explore its surface. Asteroid A minor planet. A large solid body of rock in orbit around the Sun. Banded crater A crater that displays dusky linear tracts on its inner walls and/or floor. 250 Basalt A dark, fine-grained volcanic rock, low in silicon, with a low viscosity. Basaltic material fills many of the Moon’s major basins, especially on the near side. Glossary Basin A very large circular impact structure (usually comprising multiple concentric rings) that usually displays some degree of flooding with lava. The largest and most conspicuous lava- flooded basins on the Moon are found on the near side, and most are filled to their outer edges with mare basalts. -
![Arxiv:2005.14671V2 [Astro-Ph.EP] 30 Jun 2020 Tion Et Al](https://docslib.b-cdn.net/cover/8147/arxiv-2005-14671v2-astro-ph-ep-30-jun-2020-tion-et-al-408147.webp)
Arxiv:2005.14671V2 [Astro-Ph.EP] 30 Jun 2020 Tion Et Al
Draft version July 1, 2020 Typeset using LATEX twocolumn style in AASTeX62 The Gaia-Kepler Stellar Properties Catalog. II. Planet Radius Demographics as a Function of Stellar Mass and Age Travis A. Berger,1 Daniel Huber,1 Eric Gaidos,2 Jennifer L. van Saders,1 and Lauren M. Weiss1 1Institute for Astronomy, University of Hawai`i, 2680 Woodlawn Drive, Honolulu, HI 96822, USA 2Department of Earth Sciences, University of Hawai`i at M¯anoa, Honolulu, HI 96822, USA ABSTRACT Studies of exoplanet demographics require large samples and precise constraints on exoplanet host stars. Using the homogeneous Kepler stellar properties derived using Gaia Data Release 2 by Berger et al.(2020), we re-compute Kepler planet radii and incident fluxes and investigate their distributions with stellar mass and age. We measure the stellar mass dependence of the planet radius valley to +0:21 be d log Rp/d log M? = 0:26−0:16, consistent with the slope predicted by a planet mass dependence on stellar mass (0.24{0.35) and core-powered mass-loss (0.33). We also find first evidence of a stellar age dependence of the planet populations straddling the radius valley. Specifically, we determine that the fraction of super-Earths (1{1.8 R⊕) to sub-Neptunes (1.8{3.5 R⊕) increases from 0.61 ± 0.09 at young ages (< 1 Gyr) to 1.00 ± 0.10 at old ages (> 1 Gyr), consistent with the prediction by core-powered mass- loss that the mechanism shaping the radius valley operates over Gyr timescales. Additionally, we find a tentative decrease in the radii of relatively cool (Fp < 150 F⊕) sub-Neptunes over Gyr timescales, which suggests that these planets may possess H/He envelopes instead of higher mean molecular weight atmospheres. -

Research on Crystal Growth and Characterization at the National Bureau of Standards January to June 1964
NATL INST. OF STAND & TECH R.I.C AlllDS bnSflb *^,; National Bureau of Standards Library^ 1*H^W. Bldg Reference book not to be '^sn^ t-i/or, from the library. ^ecknlccil v2ote 251 RESEARCH ON CRYSTAL GROWTH AND CHARACTERIZATION AT THE NATIONAL BUREAU OF STANDARDS JANUARY TO JUNE 1964 U. S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS tiona! Bureau of Standards NOV 1 4 1968 151G71 THE NATIONAL BUREAU OF STANDARDS The National Bureau of Standards is a principal focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. Its responsibilities include development and maintenance of the national stand- ards of measurement, and the provisions of means for making measurements consistent with those standards; determination of physical constants and properties of materials; development of methods for testing materials, mechanisms, and structures, and making such tests as may be necessary, particu- larly for government agencies; cooperation in the establishment of standard practices for incorpora- tion in codes and specifications; advisory service to government agencies on scientific and technical problems; invention and development of devices to serve special needs of the Government; assistance to industry, business, and consumers in the development and acceptance of commercial standards and simplified trade practice recommendations; administration of programs in cooperation with United States business groups and standards organizations for the development of international standards of practice; and maintenance of a clearinghouse for the collection and dissemination of scientific, tech- nical, and engineering information. The scope of the Bureau's activities is suggested in the following listing of its four Institutes and their organizational units. -

Viscosity from Newton to Modern Non-Equilibrium Statistical Mechanics
Viscosity from Newton to Modern Non-equilibrium Statistical Mechanics S´ebastien Viscardy Belgian Institute for Space Aeronomy, 3, Avenue Circulaire, B-1180 Brussels, Belgium Abstract In the second half of the 19th century, the kinetic theory of gases has probably raised one of the most impassioned de- bates in the history of science. The so-called reversibility paradox around which intense polemics occurred reveals the apparent incompatibility between the microscopic and macroscopic levels. While classical mechanics describes the motionof bodies such as atoms and moleculesby means of time reversible equations, thermodynamics emphasizes the irreversible character of macroscopic phenomena such as viscosity. Aiming at reconciling both levels of description, Boltzmann proposed a probabilistic explanation. Nevertheless, such an interpretation has not totally convinced gen- erations of physicists, so that this question has constantly animated the scientific community since his seminal work. In this context, an important breakthrough in dynamical systems theory has shown that the hypothesis of microscopic chaos played a key role and provided a dynamical interpretation of the emergence of irreversibility. Using viscosity as a leading concept, we sketch the historical development of the concepts related to this fundamental issue up to recent advances. Following the analysis of the Liouville equation introducing the concept of Pollicott-Ruelle resonances, two successful approaches — the escape-rate formalism and the hydrodynamic-mode method — establish remarkable relationships between transport processes and chaotic properties of the underlying Hamiltonian dynamics. Keywords: statistical mechanics, viscosity, reversibility paradox, chaos, dynamical systems theory Contents 1 Introduction 2 2 Irreversibility 3 2.1 Mechanics. Energyconservationand reversibility . ........................ 3 2.2 Thermodynamics. -

Facts & Features Lunar Surface Elevations Six Apollo Lunar
Greek Mythology Quadrants Maria & Related Features Lunar Surface Elevations Facts & Features Selene is the Moon and 12 234 the goddess of the Moon, 32 Diameter: 2,160 miles which is 27.3% of Earth’s equatorial diameter of 7,926 miles 260 Lacus daughter of the titans 71 13 113 Mare Frigoris Mare Humboldtianum Volume: 2.03% of Earth’s volume; 49 Moons would fit inside Earth 51 103 Mortis Hyperion and Theia. Her 282 44 II I Sinus Iridum 167 125 321 Lacus Somniorum Near Side Mass: 1.62 x 1023 pounds; 1.23% of Earth’s mass sister Eos is the goddess 329 18 299 Sinus Roris Surface Area: 7.4% of Earth’s surface area of dawn and her brother 173 Mare Imbrium Mare Serenitatis 85 279 133 3 3 3 Helios is the Sun. Selene 291 Palus Mare Crisium Average Density: 3.34 gm/cm (water is 1.00 gm/cm ). Earth’s density is 5.52 gm/cm 55 270 112 is often pictured with a 156 Putredinis Color-coded elevation maps Gravity: 0.165 times the gravity of Earth 224 22 237 III IV cresent Moon on her head. 126 Mare Marginis of the Moon. The difference in 41 Mare Undarum Escape Velocity: 1.5 miles/sec; 5,369 miles/hour Selenology, the modern-day 229 Oceanus elevation from the lowest to 62 162 25 Procellarum Mare Smythii Distances from Earth (measured from the centers of both bodies): Average: 238,856 term used for the study 310 116 223 the highest point is 11 miles. -
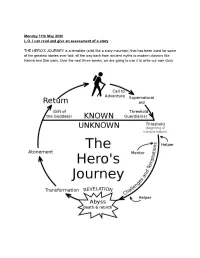
Monday 11Th May 2020 L.O. I Can Read and Give an Assessment of a Story
Monday 11th May 2020 L.O. I can read and give an assessment of a story THE HERO’S JOURNEY is a template (a bit like a story mountain) that has been used for some of the greatest stories ever told, all the way back from ancient myths to modern classics like Narnia and Star wars. Over the next three weeks, we are going to use it to write our own story One of the reasons STAR WARS is such a great movie is it because it follows the HERO’S JOURNEY model. Today, you are going to read / watch this version of the Hero’s journey and see how it fits into the format for our first act! The subtitles just refer to the stages of the story - don’t worry about them yet! Just read the story and if you have internet access look up / click on the clips on youtube. ORDINARY WORLD Luke Skywalker was a poor and humble boy who lived with his aunt and uncle in a scorching and desolate planet world called Tatooine. His job was to fix robots on the family farm and and he spent his free time flying planes through the rocky canyons. He loved his aunt and uncle but dreamed of a more exciting and adventurous life https://www.youtube.com/watch?v=8wJa1L1ZCqU Search for ‘Luke Skywalker binary sunset’ CALL TO ADVENTURE One day, Luke found a broken old R2 astromech droid. It was called R2-D2 and it was a mischievous, cheeky robot who made lots of bleeps and flashes. -

Lick Observatory Records: Photographs UA.036.Ser.07
http://oac.cdlib.org/findaid/ark:/13030/c81z4932 Online items available Lick Observatory Records: Photographs UA.036.Ser.07 Kate Dundon, Alix Norton, Maureen Carey, Christine Turk, Alex Moore University of California, Santa Cruz 2016 1156 High Street Santa Cruz 95064 [email protected] URL: http://guides.library.ucsc.edu/speccoll Lick Observatory Records: UA.036.Ser.07 1 Photographs UA.036.Ser.07 Contributing Institution: University of California, Santa Cruz Title: Lick Observatory Records: Photographs Creator: Lick Observatory Identifier/Call Number: UA.036.Ser.07 Physical Description: 101.62 Linear Feet127 boxes Date (inclusive): circa 1870-2002 Language of Material: English . https://n2t.net/ark:/38305/f19c6wg4 Conditions Governing Access Collection is open for research. Conditions Governing Use Property rights for this collection reside with the University of California. Literary rights, including copyright, are retained by the creators and their heirs. The publication or use of any work protected by copyright beyond that allowed by fair use for research or educational purposes requires written permission from the copyright owner. Responsibility for obtaining permissions, and for any use rests exclusively with the user. Preferred Citation Lick Observatory Records: Photographs. UA36 Ser.7. Special Collections and Archives, University Library, University of California, Santa Cruz. Alternative Format Available Images from this collection are available through UCSC Library Digital Collections. Historical note These photographs were produced or collected by Lick observatory staff and faculty, as well as UCSC Library personnel. Many of the early photographs of the major instruments and Observatory buildings were taken by Henry E. Matthews, who served as secretary to the Lick Trust during the planning and construction of the Observatory. -

ISSUE 134, AUGUST 2013 2 Imperative: Venus Continued
Imperative: Venus — Virgil L. Sharpton, Lunar and Planetary Institute Venus and Earth began as twins. Their sizes and densities are nearly identical and they stand out as being considerably more massive than other terrestrial planetary bodies. Formed so close to Earth in the solar nebula, Venus likely has Earth-like proportions of volatiles, refractory elements, and heat-generating radionuclides. Yet the Venus that has been revealed through exploration missions to date is hellishly hot, devoid of oceans, lacking plate tectonics, and bathed in a thick, reactive atmosphere. A less Earth-like environment is hard to imagine. Venus, Earth, and Mars to scale. Which L of our planetary neighbors is most similar to Earth? Hint: It isn’t Mars. PWhy and when did Earth’s and Venus’ evolutionary paths diverge? This fundamental and unresolved question drives the need for vigorous new exploration of Venus. The answer is central to understanding Venus in the context of terrestrial planets and their evolutionary processes. In addition, however, and unlike virtually any other planetary body, Venus could hold important clues to understanding our own planet — how it has maintained a habitable environment for so long and how long it can continue to do so. Precisely because it began so like Earth, yet evolved to be so different, Venus is the planet most likely to cast new light on the conditions that determine whether or not a planet evolves habitable environments. NASA’s Kepler mission and other concurrent efforts to explore beyond our star system are likely to find Earth-sized planets around Sun-sized stars within a few years. -

Calibration Targets
EUROPE TO THE MOON: HIGHLIGHTS OF SMART-1 MISSION Bernard H. FOING, ESA SCI-S, SMART-1 Project Scientist J.L. Josset , M. Grande, J. Huovelin, U. Keller, A. Nathues, A. Malkki, P. McMannamon, L.Iess, C. Veillet, P.Ehrenfreund & SMART-1 Science & Technology Working Team STWT M. Almeida, D. Frew, D. Koschny, J. Volp, J. Zender, RSSD & STOC G. Racca & SMART-1 Project ESTEC , O. Camino-Ramos & S1 Operations team ESOC, [email protected], http://sci.esa.int/smart-1/, www.esa.int SMART-1 project team Science Technology Working Team & ESOC Flight Control Team EUROPE TO THE MOON: HIGHLIGHTS OF SMART-1 MISSION Bernard H. Foing & SMART-1 Project & Operations team, SMART-1 Science Technology Working Team, SMART-1 Impact Campaign Team http://sci.esa.int/smart-1/, www.esa.int ESA Science programme Mars Express Smart 1 Chandrayaan1 Beagle 2 Cassini- Huygens Solar System Venus Express 05 Solar Orbiter Rosetta 04 2017 BepiColombo 2013 SMART-1 Mission SMART-1 web page (http://sci.esa.int/smart-1/) • ESA SMART Programme: Small Missions for Advanced Research in Technology – Spacecraft & payload technology demonstration for future cornerstone missions – Management: faster, smarter, better (& harder) – Early opportunity for science SMART-1 Solar Electric Propulsion to the Moon – Test for Bepi Colombo/Solar Orbiter – Mission approved and payload selected 99 – 19 kg payload (delivered August 02) – 370 kg spacecraft – launched Ariane 5 on 27 Sept 03, Kourou Europe to the Moon Some of the Innovative Technologies on Smart-1 Sun SMART-1 light Reflecte d Sun -

What Is Basic Physics Worth? François Roby
What is basic physics worth? François Roby To cite this version: François Roby. What is basic physics worth?: Orders of magnitude, energy, and overconfidence in technical refinements. 2019. hal-02004696v2 HAL Id: hal-02004696 https://hal.archives-ouvertes.fr/hal-02004696v2 Preprint submitted on 10 Feb 2019 (v2), last revised 18 Feb 2019 (v3) HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution - NonCommercial - NoDerivatives| 4.0 International License What is basic physics worth? Orders of magnitude, energy, and overconfidence in technical refinements François Roby∗ « Être informé de tout et condamné ainsi à ne rien comprendre, tel est le sort des imbéciles. » Georges Bernanos (18881948), in La France contre les robots “It doesn’t make any difference how beautiful your guess is, it doesn’t make any difference how smart you are, who made the guess, or what his name is. If it disagrees with experiment, it’s wrong. That’s all there is to it.” Richard Phillips Feynman (19181988), in a famous 1964 lecture at Cornell University. Physics is often perceived as a science of tors, the motive and the technical means are complex and precise calculations, making questioned. -

No Snowball Bifurcation on Tidally Locked Planets
Abbot Dorian - poster University of Chicago No Snowball Bifurcation on Tidally Locked Planets I'm planning to present on the effect of ocean dynamics on the snowball bifurcation for tidally locked planets. We previously found that tidally locked planets are much less likely to have a snowball bifurcation than rapidly rotating planets because of the insolation distribution. Tidally locked planets can still have a small snowball bifurcation if the heat transport is strong enough, but without ocean dynamics we did not find one in PlaSIM, an intermediate-complexity global climate model. We are now doing simulations in PlaSIM including a diffusive ocean heat transport and in the coupled ocean-atmosphere global climate model ROCKE3D to test whether ocean dynamics can introduce a snowball bifurcation for tidally locked planets. Abraham Carsten - poster University of Victoria Stable climate states and their radiative signals of Earth- like Aquaplanets Over Earth’s history Earth’s climate has gone through substantial changes such as from total or partial glaciation to a warm climate and vice versa. Even though causes for transitions between these states are not always clear one-dimensional models can determine if such states are stable. On the Earth, undoubtedly, three different stable climate states can be found: total or intermediate glaciation and ice-free conditions. With the help of a one-dimensional model we further analyse the effect of cloud feedbacks on these stable states and determine whether those stabalise the climate at different, intermediate states. We then generalise the analysis to the parameter space of a wide range of Earth-like aquaplanet conditions (changing, for instance, orbital parameters or atmospheric compositions) in order to examine their possible stable climate states and compare those to Earth’s climate states.