ASTROBIOLOGY ASTROBIOLOGY: an Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Low-Energy Lunar Trajectory Design
LOW-ENERGY LUNAR TRAJECTORY DESIGN Jeffrey S. Parker and Rodney L. Anderson Jet Propulsion Laboratory Pasadena, California July 2013 ii DEEP SPACE COMMUNICATIONS AND NAVIGATION SERIES Issued by the Deep Space Communications and Navigation Systems Center of Excellence Jet Propulsion Laboratory California Institute of Technology Joseph H. Yuen, Editor-in-Chief Published Titles in this Series Radiometric Tracking Techniques for Deep-Space Navigation Catherine L. Thornton and James S. Border Formulation for Observed and Computed Values of Deep Space Network Data Types for Navigation Theodore D. Moyer Bandwidth-Efficient Digital Modulation with Application to Deep-Space Communication Marvin K. Simon Large Antennas of the Deep Space Network William A. Imbriale Antenna Arraying Techniques in the Deep Space Network David H. Rogstad, Alexander Mileant, and Timothy T. Pham Radio Occultations Using Earth Satellites: A Wave Theory Treatment William G. Melbourne Deep Space Optical Communications Hamid Hemmati, Editor Spaceborne Antennas for Planetary Exploration William A. Imbriale, Editor Autonomous Software-Defined Radio Receivers for Deep Space Applications Jon Hamkins and Marvin K. Simon, Editors Low-Noise Systems in the Deep Space Network Macgregor S. Reid, Editor Coupled-Oscillator Based Active-Array Antennas Ronald J. Pogorzelski and Apostolos Georgiadis Low-Energy Lunar Trajectory Design Jeffrey S. Parker and Rodney L. Anderson LOW-ENERGY LUNAR TRAJECTORY DESIGN Jeffrey S. Parker and Rodney L. Anderson Jet Propulsion Laboratory Pasadena, California July 2013 iv Low-Energy Lunar Trajectory Design July 2013 Jeffrey Parker: I dedicate the majority of this book to my wife Jen, my best friend and greatest support throughout the development of this book and always. -
The Influence of Electronegativity on Linear and Triangular Three-Centre Bonds
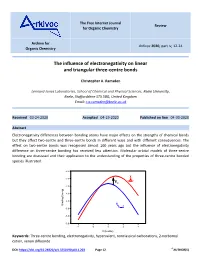
The Free Internet Journal Review for Organic Chemistry Archive for Arkivoc 2020, part iv, 12-24 Organic Chemistry The influence of electronegativity on linear and triangular three-centre bonds Christopher A. Ramsden Lennard-Jones Laboratories, School of Chemical and Physical Sciences, Keele University, Keele, Staffordshire ST5 5BG, United Kingdom Email: [email protected] Received 03-24-2020 Accepted 04-19-2020 Published on line 04-30-2020 Abstract Electronegativity differences between bonding atoms have major effects on the strengths of chemical bonds but they affect two-centre and three-centre bonds in different ways and with different consequences. The effect on two-centre bonds was recognised almost 100 years ago but the influence of electronegativity difference on three-centre bonding has received less attention. Molecular orbital models of three-centre bonding are discussed and their application to the understanding of the properties of three-centre bonded species illustrated. -2.5 X -3.0 + Y Y Ea -3.5 -4.0 -4.5 X Binding Energy Binding + -5.0 Y Y -5.5 -6.0 -4 -2 0 2 4 h (b units) Keywords: Three-centre bonding, electronegativity, hypervalent, nonclassical carbocations, 2-norbornyl cation, xenon difluoride DOI: https://doi.org/10.24820/ark.5550190.p011.203 Page 12 ©AUTHOR(S) Arkivoc 2020, iv, 12-24 Ramsden, C. A. Table of Contents 1. Introduction 2. Linear Three-Centre Bonds (Hypervalent Bonds) (X-Y-X) X 3. Triangular Three-Centre Bonds (Y Y) 4. The Localised Bond Model of Two- and Three-Centre Bonds 5. Conclusions References -

Introduction to Astronomy from Darkness to Blazing Glory
Introduction to Astronomy From Darkness to Blazing Glory Published by JAS Educational Publications Copyright Pending 2010 JAS Educational Publications All rights reserved. Including the right of reproduction in whole or in part in any form. Second Edition Author: Jeffrey Wright Scott Photographs and Diagrams: Credit NASA, Jet Propulsion Laboratory, USGS, NOAA, Aames Research Center JAS Educational Publications 2601 Oakdale Road, H2 P.O. Box 197 Modesto California 95355 1-888-586-6252 Website: http://.Introastro.com Printing by Minuteman Press, Berkley, California ISBN 978-0-9827200-0-4 1 Introduction to Astronomy From Darkness to Blazing Glory The moon Titan is in the forefront with the moon Tethys behind it. These are two of many of Saturn’s moons Credit: Cassini Imaging Team, ISS, JPL, ESA, NASA 2 Introduction to Astronomy Contents in Brief Chapter 1: Astronomy Basics: Pages 1 – 6 Workbook Pages 1 - 2 Chapter 2: Time: Pages 7 - 10 Workbook Pages 3 - 4 Chapter 3: Solar System Overview: Pages 11 - 14 Workbook Pages 5 - 8 Chapter 4: Our Sun: Pages 15 - 20 Workbook Pages 9 - 16 Chapter 5: The Terrestrial Planets: Page 21 - 39 Workbook Pages 17 - 36 Mercury: Pages 22 - 23 Venus: Pages 24 - 25 Earth: Pages 25 - 34 Mars: Pages 34 - 39 Chapter 6: Outer, Dwarf and Exoplanets Pages: 41-54 Workbook Pages 37 - 48 Jupiter: Pages 41 - 42 Saturn: Pages 42 - 44 Uranus: Pages 44 - 45 Neptune: Pages 45 - 46 Dwarf Planets, Plutoids and Exoplanets: Pages 47 -54 3 Chapter 7: The Moons: Pages: 55 - 66 Workbook Pages 49 - 56 Chapter 8: Rocks and Ice: -

Download This Article in PDF Format
BIO Web of Conferences 4, 00014 (2015) DOI: 10.1051/bioconf/20150400014 C Owned by the authors, published by EDP Sciences, 2015 From neonts to filionts and their progenies... Biodiversity draws its origins from the origins of life itself Marie-Christine Maurela Institut de Systématique, Évolution, Biodiversité (ISYEB - UMR 7205 – CNRS, MNHN, UPMC, EPHE), Muséum national d’Histoire naturelle, Sorbonne Universités, 45 rue Buffon, CP. 50, 75005 Paris, France “Should the question of the priority of the egg over the chicken, or of the chicken over the egg disturb you, it is because you assume that animals were originally what they are to-day; what madness!" The dream of d’Alembert, 1760, Denis Diderot Abstract. Since the origins, different modes of life exist. Indeed it is difficult to imagine how things could have been constructed by a single event that would have marked the birth of life. A brief historical background of the subject will be presented here. Then, we shall wander through the history of the discoveries that have led the way of experimental science over the last 150 years and shall dwell on one of the major paradigms, that of the RNA World. This paradigm illustrates the path followed by the living that tries all possible means when expressed at the molecular level as simple free molecule leading to RNA fragments and to modern subviral particles such as viroids then to ramifications and compartments. Between the ancient and the modern RNA world we underline how environment talks to molecules leading to the global living tissus which establish the ground of biodiversity. -
![Arxiv:2005.14671V2 [Astro-Ph.EP] 30 Jun 2020 Tion Et Al](https://docslib.b-cdn.net/cover/8147/arxiv-2005-14671v2-astro-ph-ep-30-jun-2020-tion-et-al-408147.webp)
Arxiv:2005.14671V2 [Astro-Ph.EP] 30 Jun 2020 Tion Et Al
Draft version July 1, 2020 Typeset using LATEX twocolumn style in AASTeX62 The Gaia-Kepler Stellar Properties Catalog. II. Planet Radius Demographics as a Function of Stellar Mass and Age Travis A. Berger,1 Daniel Huber,1 Eric Gaidos,2 Jennifer L. van Saders,1 and Lauren M. Weiss1 1Institute for Astronomy, University of Hawai`i, 2680 Woodlawn Drive, Honolulu, HI 96822, USA 2Department of Earth Sciences, University of Hawai`i at M¯anoa, Honolulu, HI 96822, USA ABSTRACT Studies of exoplanet demographics require large samples and precise constraints on exoplanet host stars. Using the homogeneous Kepler stellar properties derived using Gaia Data Release 2 by Berger et al.(2020), we re-compute Kepler planet radii and incident fluxes and investigate their distributions with stellar mass and age. We measure the stellar mass dependence of the planet radius valley to +0:21 be d log Rp/d log M? = 0:26−0:16, consistent with the slope predicted by a planet mass dependence on stellar mass (0.24{0.35) and core-powered mass-loss (0.33). We also find first evidence of a stellar age dependence of the planet populations straddling the radius valley. Specifically, we determine that the fraction of super-Earths (1{1.8 R⊕) to sub-Neptunes (1.8{3.5 R⊕) increases from 0.61 ± 0.09 at young ages (< 1 Gyr) to 1.00 ± 0.10 at old ages (> 1 Gyr), consistent with the prediction by core-powered mass- loss that the mechanism shaping the radius valley operates over Gyr timescales. Additionally, we find a tentative decrease in the radii of relatively cool (Fp < 150 F⊕) sub-Neptunes over Gyr timescales, which suggests that these planets may possess H/He envelopes instead of higher mean molecular weight atmospheres. -

Bun)Mo(Pme3)(Η -C2H4) (6) Through Etmgbr Reduction of (Arn)( Bun)Mocl2(DME) in the Presence of Pme3
Synthesis, Catalysis and Stoichiometric Reactivity of Mo Imido Complexes Nicolas A. McLeod BSc. in Chemistry Chemistry Thesis submitted to the Faculty of Graduate Studies In the partial fulfillment of the requirements For the MSc. degree in chemistry Chemistry Department Faculty of Mathematics and Science Brock University St. Catharines, Ontario © Nicolas A. McLeod, 2014 Abstract The current thesis describes the syntheses, catalytic reactivity and mechanistic investigations of novel Mo(IV) and Mo(VI) imido systems. Attempts at preparing mixed bis(imido) Mo(IV) complexes of the type (RN)(R′N)Mo(PMe3)n (n = 2 or 3) derived from the t mono(imido) complexes (RN)Mo(PMe3)3(X)2 (R = Bu (1) or Ar (2); X = Cl2 or HCl, Ar=2,6- i Pr2C6H3) are also described. The addition of lithiated silylamides to 1 or 2 results in the 2 unexpected formation of the C-H activated cyclometallated complexes (RN)Mo(PMe3)2(η - t CH2PMe2)(X) (R = Ar, X = H (3); R = Bu, X = Cl (4)). Complexes 3 and 4 were used in the activation of R′E-H bonds (E = Si, B, C, O, P; R′ = alkyl or aryl), which typically give products of addition across the M-C bond of the type (RN)Mo(PMe3)3(ER′)(X) (4). In the case of 2,6- dimethylphenol, subsequent heating of 4 (R = Ar, R′ = 2,6-Me2C6H3, E = O) to 50 °C results in 2 C-H activation to give the cyclometallated complex (ArN)Mo(PMe3)3(κ -O,C-OPh(Me)CH2) (5). An alternative approach was developed in synthesizing the mixed imido complex t 2 t (ArN)( BuN)Mo(PMe3)(η -C2H4) (6) through EtMgBr reduction of (ArN)( BuN)MoCl2(DME) in the presence of PMe3. -

One-Armed Oscillations in Be Star Discs
A&A 456, 1097–1104 (2006) Astronomy DOI: 10.1051/0004-6361:20065407 & c ESO 2006 Astrophysics One-armed oscillations in Be star discs J. C. B. Papaloizou1 andG.J.Savonije2 1 Department of Applied Mathematics and Theoretical Physics, Centre for Mathematical Sciences, Wilberforce Road, Cambridge CB3 0WA, UK 2 Astronomical Institute “Anton Pannekoek”, University of Amsterdam, Kruislaan 403, 1098 SJ Amsterdam, The Netherlands e-mail: [email protected] Received 11 April 2006 / Accepted 20 June 2006 ABSTRACT Aims. In this paper we study the effect of the quadrupole-term in the gravitational potential of a rotationally deformed central Be star on one armed density waves in the circumstellar disc. The aim is to explain the observed long-term violet over red (V/R) intensity variations of the double peaked Balmer emission-lines, not only in cool Be star systems, but also in the hot systems like γ Cas. Methods. We have carried out semi-analytic and numerical studies of low-frequency one armed global oscillations in near Keplerian discs around Be stars. In these we have investigated surface density profiles for the circumstellar disc which have inner narrow low surface density or gap regions, just interior to global maxima close to the rapidly rotating star, as well as the mode inner boundary conditions. Results. Our results indicate that it is not necessary to invoke extra forces such as caused by line absorption from the stellar flux in order to explain the long-term V/R variations in the discs around massive Be stars. When there exists a narrow gap between the star and its circumstellar disc, with the result that the radial velocity perturbation is non-zero at the inner disc boundary, we find oscillation (and V/R) periods in the observed range for plausible magnitudes for the rotational quadrupole term. -

Preliminary JIRAM Results from Juno Polar Observations: 2. Analysis Of
PUBLICATIONS Geophysical Research Letters RESEARCH LETTER Preliminary JIRAM results from Juno polar observations: 10.1002/2017GL072905 + 2. Analysis of the Jupiter southern H3 emissions Special Section: and comparison with the north aurora Early Results: Juno at Jupiter A. Adriani1 , A. Mura1 , M. L. Moriconi2 , B. M. Dinelli2 , F. Fabiano2,3 , F. Altieri1 , G. Sindoni1 , S. J. Bolton4 , J. E. P. Connerney5 , S. K. Atreya6, F. Bagenal7 , Key Points: + 8 1 1 1 1 1 • H3 intensity, column density, and J.-C. M. C. Gérard , G. Filacchione , F. Tosi , A. Migliorini , D. Grassi , G. Piccioni , temperature maps of the Jupiter R. Noschese1, A. Cicchetti1 , G. R. Gladstone4, C. Hansen9 , W. S. Kurth10 , S. M. Levin11 , southern aurora are derived from B. H. Mauk12 , D. J. McComas13 , A. Olivieri14, D. Turrini1 , S. Stefani1 , and M. Amoroso14 Juno/JIRAM data collected on the first orbit 1INAF-Istituto di Astrofisica e Planetologia Spaziali, Rome, Italy, 2CNR-Istituto di Scienze dell’Atmosfera e del Clima, Bologna, • Emissions from southern aurora are 3 4 more intense than from the North Italy, Dipartimento di Fisica e Astronomia, Università di Bologna, Bologna, Italy, Space Science Department, Southwest • Derived temperatures are in the range Research Institute, San Antonio, Texas, USA, 5Solar System Exploration Division, Planetary Magnetospheres Laboratory, 600°K to 1400°K NASA Goddard Space Flight Center, Greenbelt, Maryland, USA, 6Planetary Science Laboratory, University of Michigan, Ann Arbor, Michigan, USA, 7Laboratory for Atmospheric and Space Physics, University of Colorado Boulder, Boulder, Colorado, USA, 8Laboratoire de Physique Atmosphérique et Planétaire, University of Liège, Liège, Belgium, 9Planetary Science 10 Correspondence to: Institute, Tucson, Arizona, USA, Jet Propulsion Laboratory, California Institute of Technology, Pasadena, California, USA, 11 12 A. -

Detection of Nitrogen Gas in the Β Pictoris Circumstellar Disc P
Manuscript version: Published Version The version presented in WRAP is the published version (Version of Record). Persistent WRAP URL: http://wrap.warwick.ac.uk/110773 How to cite: The repository item page linked to above, will contain details on accessing citation guidance from the publisher. Copyright and reuse: The Warwick Research Archive Portal (WRAP) makes this work by researchers of the University of Warwick available open access under the following conditions. Copyright © and all moral rights to the version of the paper presented here belong to the individual author(s) and/or other copyright owners. To the extent reasonable and practicable the material made available in WRAP has been checked for eligibility before being made available. Copies of full items can be used for personal research or study, educational, or not-for-profit purposes without prior permission or charge. Provided that the authors, title and full bibliographic details are credited, a hyperlink and/or URL is given for the original metadata page and the content is not changed in any way. Publisher’s statement: Please refer to the repository item page, publisher’s statement section, for further information. For more information, please contact the WRAP Team at: [email protected] warwick.ac.uk/lib-publications A&A 621, A121 (2019) Astronomy https://doi.org/10.1051/0004-6361/201834346 & © ESO 2019 Astrophysics Detection of nitrogen gas in the β Pictoris circumstellar disc P. A. Wilson1,2,3,4,5, R. Kerr6, A. Lecavelier des Etangs4,5, V. Bourrier4,5,7, A. Vidal-Madjar4,5, F. Kiefer4,5, and I. -

Finding a New Route to the Moon Using Paintings
Bridges 2009: Mathematics, Music, Art, Architecture, Culture Finding a New Route to the Moon using Paintings Edward Belbruno Department of Astrophysical Sciences Princeton University Princeton, NJ, 08540, USA E-mail: [email protected] Abstract A new theory of space travel using low energy chaotic trajectories was dramatically demonstrated in 1991 with the rescue of a Japanese lunar mission, getting its spacecraft, Hiten, to the Moon on a different type of route. The design of this route and the theory behind it is based on finding regions of instability about the Moon. This approach to space travel was inspired by a painting of the Earth-Moon system which revealed these regions and the new chaotic trajectories. The brush strokes in the painting revealed the dynamics of motion which are verified by the computer. The painting itself can be used as a way to uncover dynamics in the field of celestial mechanics that cannot be easily found my standard mathematical methods. A New Approach to Space Travel The classical route from the Earth to the Moon uses the Hohmann transfer, named after the German engineer Walter Hohmann [1]. Let’s assume a spacecraft is to go from a circular orbit about the Earth to a circular orbit about the Moon. The Hohmann transfer is a nearly straight path, actually half of a highly eccentric ellipse, from the circular orbit about the Earth to the circular orbit about the Moon. To leave the orbit about the Earth, the spacecraft briefly fires its engines to gain the necessary velocity, then coasts to the Moon in three days. -

Gas Accretion by Giant Planets : a Study with 3D Inviscid Hydrodynamical Simulations
EPSC Abstracts Vol. 8, EPSC2013-363, 2013 European Planetary Science Congress 2013 EEuropeaPn PlanetarSy Science CCongress c Author(s) 2013 Gas Accretion by Giant Planets : a study with 3D inviscid hydrodynamical simulations J. Szulágyi (1), A. Morbidelli (1), A. Crida (1) and F. Masset (2) (1) University of Nice-Sophia Antipolis, CNRS, Observatoire de la Côte d’Azur, Laboratoire Lagrange, France ([email protected]) (2) Institute of Physical Sciences, Universidad Nacional Autónoma de México Abstract If this were true, there should be a dichotomy in the mass distribution of planets : planets should be either We investigate the properties of the circumplanetary smaller than 30 Earth masses (those that did not ∼ disc (CPD) of a Jupiter-mass planet with a three- reach the phase of runaway gas accretion) or of mul- dimensional hydrodynamical nested grid code. We tiple Jupiter-masses (those that entered and completed perform isothermal simulations of a large radial por- the fast runaway gas accretion). Planets in between tion of the circumstellar disc and, with the help of a these two mass categories should be extremely rare, system of 8 nested grids, we zoom into the planet’s conversely to what is observed [4]. vicinity. Since giant planets are thought to form in a A very likely possibility, however, is that the cir- dead zone, we do not use any prescribed viscosity in cumplanetary disc acts as a regulator of the rate of gas the fluid-dynamics equations. accretion onto the planet. In particular, if the circum- We discuss in details the geometry of the circum- planetary disc (CPD) has a very low viscosity [8], then planetary disc, and especially focus on the role of the the transport of angular momentum through this disc polar inflow. -
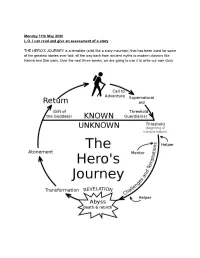
Monday 11Th May 2020 L.O. I Can Read and Give an Assessment of a Story
Monday 11th May 2020 L.O. I can read and give an assessment of a story THE HERO’S JOURNEY is a template (a bit like a story mountain) that has been used for some of the greatest stories ever told, all the way back from ancient myths to modern classics like Narnia and Star wars. Over the next three weeks, we are going to use it to write our own story One of the reasons STAR WARS is such a great movie is it because it follows the HERO’S JOURNEY model. Today, you are going to read / watch this version of the Hero’s journey and see how it fits into the format for our first act! The subtitles just refer to the stages of the story - don’t worry about them yet! Just read the story and if you have internet access look up / click on the clips on youtube. ORDINARY WORLD Luke Skywalker was a poor and humble boy who lived with his aunt and uncle in a scorching and desolate planet world called Tatooine. His job was to fix robots on the family farm and and he spent his free time flying planes through the rocky canyons. He loved his aunt and uncle but dreamed of a more exciting and adventurous life https://www.youtube.com/watch?v=8wJa1L1ZCqU Search for ‘Luke Skywalker binary sunset’ CALL TO ADVENTURE One day, Luke found a broken old R2 astromech droid. It was called R2-D2 and it was a mischievous, cheeky robot who made lots of bleeps and flashes.