PHENOLOGY, BIOMETRICS and FRUITS PRODUCTION of Attalea
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

OFI Jan19 P27,28,29 NEW.Indd 2 17/12/2018 11:50:37 PALM OIL
PALM OIL High oleic palm oil has a lower saturate value than conventional palm oil e high oleic option A hybrid form of palm oil grown in Colombia and Ecuador Production of the oil will grow even more, from 84,278 tonnes in 2013 to a has a higher oleic content than conventional palm oil, oering forecast 428,501 tonnes in 2020. applications in frying, baking and chocolate spreads HOPO has a higher production cost, due to the need for assisted pollination, Jose Angel Olivero but research is currently underway to address this. It also has a lower amount igh oleic palm oil (HOPO) is a non from the OXG hybrid contains more than of kernel and therefore less palm kernel GMO hybrid, known as Oleifera X 50% oleic acid, 33% SAFA and 12% oil. But in areas where bud rot is endemic, HGuineensis (OXG). It was developed PUFA, a very balanced fatty acid profile. growing HOPO is an alternative as it is and introduced in Colombia, Ecuador and HOPO is chiefly grown in Colombia tolerant to the disease. other countries in the 1990s as a result of and Ecuador, where the planted area is Physically, HOPO is more liquid at room a cross between the male Elaeis guineensis, projected to nearly triple from 55,033ha temperature than conventional palm (the conventional palm tree from the Gulf in 2013 to 153,400ha in 2020 (see Table oil as it has a different solid fat content of Guinea in Africa) and the female Elaeis 1, following page). (SFC) curve derived from its different u oleifera, originally from tropical South America, including the Amazon Basin of Brazil, Colombia and other countries in the region. -

The Oil Palm (Elaeis Guineensis)
PALM S Rival & Levang: Oil Palm Vol. 59(1) 2015 ALAIN RIVAL The Oil Palm Centre de Coopération Internationale en Recherche (Elaeis Agronomique pour le Développement guineensis ): Jakarta, Indonesia [email protected] Research AND Challenges PATRICE LEVANG Institut de Recherche pour Beyond le Développement Yaoundé, Cameroon Controversies [email protected] Scientists certainly have a part to play in the debate over oil palm ( Elaeis guineensis Jacq.) cultivation, which has captured and polarized public opinion, kindled and undoubtedly shaped by the media. How can this palm be viewed as a “miracle plant” by both the agro-food industry in the North and farmers in the tropical zone, but a serious ecological threat by non-governmental organizations (NGOs) campaigning for the environment or the rights of indigenous peoples? The time has come to move on from this biased and often irrational debate, which is rooted in topical issues of contemporary society in the North, such as junk food, biodiversity, energy policy and ethical consumption. One of the reasons the public has developed as nuclear energy, genetically modified crops such fixed ideas is that there has been a lack or shale gas) that is causing controversy but an of accurate information on the sector and its entire agrom-food sector that has come to actors and a clear-headed analysis of what is symbolize the conflict between the at stake. We point out that the production and conservation of natural spaces and de- processing of palm oil are part of a complex velopment. Consumers, elected representatives globalized agrom-industrial sector shared by and scientists are finally forced to take sides for multiple actors and stakeholders with often or against palm oil, with no room for ifs and conflicting interests. -
![Syagrus Romanzoffiana [Cham.] Glassman](https://docslib.b-cdn.net/cover/5715/syagrus-romanzoffiana-cham-glassman-115715.webp)
Syagrus Romanzoffiana [Cham.] Glassman
SCIENTIFIC note Doi: https://doi.org/10.17584/rcch.2019v13i3.8363 Pre-depulping and depulping treatments and the emergence of queen palm seeds (Syagrus romanzoffiana [Cham.] Glassman) Tratamiento de pre-despulpado y despulpado sobre la emergencia de semillas de palma reina (Syagrus romanzoffiana [Cham.] Glassman) LUCAS MARQUEZAN NASCIMENTO1 EDUARDO PRADI VENDRUSCOLO2, 4 LUIZ FERNANDES CARDOSO CAMPOS1 LISMAÍRA GONÇALVES CAIXETA GARCIA1 LARISSA LEANDRO PIRES1 ALEXANDER SELEGUINI3 Syagrus romanzoffiana under conditions of Brazilian Cerrado. Photo: L.M. Nascimento ABSTRACT The propagation of the palm Syagrus romanzoffiano is done sexually with seeds, making the process of obtai- ning new plants slow and difficult, especially on large scales. In addition, seed germination is slow, uneven and susceptible to degradation and loss of vigor because of embryo deterioration, even under laboratory conditions. As a result of the lack of information on efficient depulping methods for queen palm fruits, the present study aimed to establish a depulping methodology that is less aggressive to embryos, maintaining emergence quality. This experiment was carried out in Goiânia, Brazil, using fruits from eight stock plants submitted to three pre-depulping treatments (control, fermentation and drying) and two depulping me- thods (industrial depulping and concrete-mixer with the addition of gravel). After the different pre-sowing processes, the fresh and dry pyrenes mass, remaining fibers adhered to the pyrene and seedling emergence were evaluated. The pulper removed an average of 45% more pyrene pulp than the concrete mixer. However, these methodologies did not result in differences in the emergence of plants, which was affected only by the pre-depulping treatment, with superiority in the use of fresh fruits. -

Nölken Palm(Kernel)Oil-Statement
2 Nölken Palm oil and palm kernel oil Statement Palm oil is one of the most important vegetable oils as well as the displacement of indigenous people and in the world and is used in many consumer goods. the destruction of biodiversity. During the extraction of palm oil from the fruit, it is also possible to obtain palm kernel oil. This oil from For the variety of care and cosmetics products which palm kernel is a key ingredient for the production of we produce, we use raw materials such as surfactants washing and cleaning substances, e.g. for cosmetics and or emulsifiers based on renewable raw materials with detergents. Palm oil is also used in the food industry and palm kernel oil for example as a primary material. These as fuels or combustibles. However, the cultivation of oil raw materials are identified as palm oil or palm kernel palms (Elaeis guineensis) is often criticised because the oil derivatives. As a result of their productivity, palm production of palm oil is still associated with negative kernel oil derivatives are best suited to the production effects such as the clearance of rain forests, cultivation of cosmetic products. on peat soil with the emission of large amounts of CO2, Replacing palm kernel oil with other oils is not really a non-governmental organisations (i.e. WWF, Greenpea- solution. The shift to soy oil for example, the second ce) call not for an end to the use of palm (kernel) oil most important vegetable oil in the world, would then but for a transfer to a sustainable cultivation of palm cause problems in other countries. -

Current Knowledge on Interspecific Hybrid Palm Oils As Food and Food
foods Review Current Knowledge on Interspecific Hybrid Palm Oils as Food and Food Ingredient Massimo Mozzon , Roberta Foligni * and Cinzia Mannozzi * Department of Agricultural, Food and Environmental Sciences, Università Politecnica delle Marche, Via Brecce Bianche 10, 60131 Ancona, Italy; m.mozzon@staff.univpm.it * Correspondence: r.foligni@staff.univpm.it (R.F.); c.mannozzi@staff.univpm.it (C.M.); Tel.: +39-071-220-4010 (R.F.); +39-071-220-4014 (C.M.) Received: 6 April 2020; Accepted: 10 May 2020; Published: 14 May 2020 Abstract: The consumers’ opinion concerning conventional palm (Elaeis guineensis) oil is negatively affected by environmental and nutritional issues. However, oils extracted from drupes of interspecific hybrids Elaeis oleifera E. guineensis are getting more and more interest, due to their chemical and × nutritional properties. Unsaturated fatty acids (oleic and linoleic) are the most abundant constituents (60%–80% of total fatty acids) of hybrid palm oil (HPO) and are mainly acylated in position sn-2 of the glycerol backbone. Carotenes and tocotrienols are the most interesting components of the unsaponifiable matter, even if their amount in crude oils varies greatly. The Codex Committee on Fats and Oils recently provided HPO the “dignity” of codified fat substance for human consumption and defined the physical and chemical parameters for genuine crude oils. However, only few researches have been conducted to date on the functional and technological properties of HPO, thus limiting its utilization in food industry. Recent studies on the nutritional effects of HPO softened the initial enthusiasm about the “tropical equivalent of olive oil”, suggesting that the overconsumption of HPO in the most-consumed processed foods should be carefully monitored. -

Oenocarpus Bataua Mart.): a Palm from the Amazon
International Journal of Plant & Soil Science 31(6): 1-7, 2019; Article no.IJPSS.54389 ISSN: 2320-7035 Mineralogical Composition and Bioactive Molecules in the Pulp and Seed of Patauá (Oenocarpus bataua Mart.): A Palm from the Amazon S. A. M. Saravia1*, I. F. Montero2, B. M. Linhares3, R. A. Santos4 and J. A. F. Marcia5 1Faculty of Earth Sciences and Conservation, National University of Agriculture, Highway to Dulce Nombre de Culmi, Km 215, Neighborhood El Espino, Catacamas-Olancho, Honduras. 2Department of Organic and Inorganic Chemistry, Polytechnic School, University of Extremadura, University Avenue s/n, Cáceres, Spain. 3Federal Institute of Roraima, Campus Boa Vista, Av. Glaycon de Paiva, 2496 - Pricumã, Boa Vista - RR, 69303-340, Brazil. 4Department of Food Science, Louisiana State University, USA. 5Faculty of Technological Sciences, National University of Agriculture, Highway to Dulce Nombre de Culmi, Km 215, Neighborhood El Espino, Catacamas-Olancho, Honduras. Authors’ contributions This work was carried out in collaboration among all authors. Author SAMS contributed to the analysis, literature review and formatting of the article. Author IFM contributed to the supervision of the analysis, writing of the article and review of language and style. Author BML contributed to the collection of the material, sample preparation and analysis. Authors RAS and JAFM contributed to the review, editing of the article, the verification, validation of the methods and analysis being used in the experiments. All authors read and approved the final manuscript. Article Information DOI: 10.9734/IJPSS/2019/v31i630228 Editor(s): (1) Dr. Olanrewaju Folusho Olotuah, Professor, Department of Plant Science and Biotechnology, Adekunle Ajasin University, Akungba-Akoko, Ondo State, Nigeria. -

Middle to Late Paleocene Leguminosae Fruits and Leaves from Colombia
AUTHORS’ PAGE PROOFS: NOT FOR CIRCULATION CSIRO PUBLISHING Australian Systematic Botany https://doi.org/10.1071/SB19001 Middle to Late Paleocene Leguminosae fruits and leaves from Colombia Fabiany Herrera A,B,D, Mónica R. Carvalho B, Scott L. Wing C, Carlos Jaramillo B and Patrick S. Herendeen A AChicago Botanic Garden, 1000 Lake Cook Road, Glencoe, IL 60022, USA. BSmithsonian Tropical Research Institute, Box 0843-03092, Balboa, Ancón, Republic of Panamá. CDepartment of Paleobiology, NHB121, PO Box 37012, Smithsonian Institution, Washington, DC 20013, USA. DCorresponding author. Email: [email protected] Abstract. Leguminosae are one of the most diverse flowering-plant groups today, but the evolutionary history of the family remains obscure because of the scarce early fossil record, particularly from lowland tropics. Here, we report ~500 compression or impression specimens with distinctive legume features collected from the Cerrejón and Bogotá Formations, Middle to Late Paleocene of Colombia. The specimens were segregated into eight fruit and six leaf 5 morphotypes. Two bipinnate leaf morphotypes are confidently placed in the Caesalpinioideae and are the earliest record of this subfamily. Two of the fruit morphotypes are placed in the Detarioideae and Dialioideae. All other fruit and leaf morphotypes show similarities with more than one subfamily or their affinities remain uncertain. The abundant fossil fruits and leaves described here show that Leguminosae was the most important component of the earliest rainforests in northern South America c. 60–58 million years ago. Additional keywords: diversity, Fabaceae, fossil plants, legumes, Neotropics, South America. Received 10 January 2019, accepted 5 April 2019, published online dd mmm yyyy Introduction dates for the crown clades ranging from the Cretaceous to the – Leguminosae, the third-largest family of flowering plants with Early Paleogene, c. -
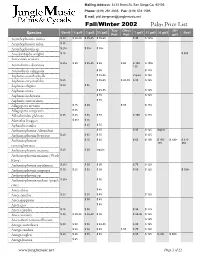
Winter-Fall Sale 2002 Palm Trees-Web
Mailing Address: 3233 Brant St. San Diego Ca, 92103 Phone: (619) 291 4605 Fax: (619) 574 1595 E mail: [email protected] Fall/Winter 2002 Palm Price List Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Acanthophoenix crinita $ 30 $ 30-40 $ 35-45 $ 55-65 $ 95 $ 125+ Acanthophoenix rubra $ 35 Acanthophoenix sp. $ 25+ $ 35+ $ 55+ Acoelorrhaphe wrightii $ 15 $ 300 Acrocomia aculeata $ 25+ $ 35 $ 35-45 $ 65 $ 65 $ 100- $ 150+ Actinokentia divaricata 135 Actinorhytis calapparia $ 55 $ 125 Aiphanes acanthophylla $ 45-55 inquire $ 125 Aiphanes caryotaefolia $ 25 $ 55-65 $ 45-55 $ 85 $ 125 Aiphanes elegans $ 20 $ 35 Aiphanes erosa $ 45-55 $ 125 Aiphanes lindeniana $ 55 $ 125 Aiphanes vincentsiana $ 55 Allagoptera arenaria $ 25 $ 40 $ 55 $ 135 Allagoptera campestris $ 35 Alloschmidtia glabrata $ 35 $ 45 $ 55 $ 85 $ 150 $ 175 Alsmithia longipes $ 35+ $ 55 Aphandra natalia $ 35 $ 55 Archontophoenix Alexandrae $ 55 $ 85 $ 125 inquire Archontophoenix Beatricae $ 20 $ 35 $ 55 $ 125 Archontophoenix $ 25 $ 45 $ 65 $ 100 $ 150- $ 200+ $ 310- 175 350 cunninghamiana Archontophoenix maxima $ 25 $ 30 inquire Archontophoenix maxima (Wash River) Archontophoenix myolaensis $ 25+ $ 30 $ 50 $ 75 $ 125 Archontophoenix purpurea $ 30 $ 25 $ 35 $ 50 $ 85 $ 125 $ 300+ Archontophoenix sp. Archontophoenix tuckerii (peach $ 25+ $ 55 river) Areca alicae $ 45 Areca catechu $ 20 $ 35 $ 45 $ 125 Areca guppyana $ 30 $ 45 Areca ipot $ 45 Areca triandra $ 25 $ 30 $ 95 $ 125 Areca vestiaria $ 25 $ 30-35 $ 35-40 $ 55 $ 85-95 $ 125 Arecastrum romanzoffianum $ 125 Arenga australasica $ 20 $ 30 $ 35 $ 45-55 $ 85 $ 125 Arenga caudata $ 20 $ 30 $ 45 $ 55 $ 75 $ 100 Arenga engleri $ 20 $ 60 $ 35 $ 45 $ 85 $ 125 $ 200 $ 300+ Arenga hastata $ 25 www.junglemusic.net Page 1 of 22 Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Arenga hookeriana inquire Arenga micranthe 'Lhutan' $ 20 inquire Arenga pinnata $ 35 $ 50 $ 85 $ 125 Arenga sp. -

Confederated Tribes of the Umatilla Indian Reservation P.O
Revised CTUIR RENEWABLE ENERGY FEASIBILITY STUDY FINAL REPORT June 20, 2005 Rev.October 31, 2005 United States Government Department of Energy National Renewable Energy Laboratory DE-FC36-02GO-12106 Compiled under the direction of: Stuart G. Harris, Director Department of Science & Engineering Confederated Tribes of the Umatilla Indian Reservation P.O. Box 638 Pendleton, Oregon 97801 2 Table of Contents Page No. I. Acknowledgement 5 II. Summary 6 III. Introduction 12 III-1. CTUIR Energy Uses and Needs 14 III-1-1. Residential Population – UIR 14 III-1-2. Residential Energy Use – UIR 14 III-1-3. Commercial and Industrial Energy Use – UIR 15 III-1-4. Comparison of Energy Cost on UIR with National Average 16 III-1-5. Petroleum and Transportation Energy Usage 16 III-1-6. Electrical Power Needs – UIR 17 III-1-7. State of Oregon Energy Consumption Statistics 17 III-1-8 National Energy Outlook 17 III-2. Energy Infrastructure on Umatilla Indian Reservation 19 III-2-1. Electrical 20 III-2-2. Natural Gas 21 III-2-3. Biomass Fuels 21 III-2-4. Transportation Fuels 21 III-2-5. Other Energy Sources 21 III-3. Renewable Energy Economics 21 III-3-1. Financial Figures of Merit 21 III-3-2. Financial Structures 22 III-3-3. Calculating Levelized Cost of Energy (COE) 23 III-3-4. Financial Model and Results 25 IV. Renewable Energy Resources, Technologies and Economics – In-and-Near the UIR 27 IV-1 Biomass Resources 27 IV-1-1. Resource Availability 27 IV-1-1-1. Forest Residues 27 IV-1-1-2. -

Estimating Babassu Palm Density Using Automatic Palm Tree Detection
Estimating babassu palm density using automatic palm tree detection with very high spatial resolution satellite images Alessio Moreira dos Santos, Danielle Mitja, Eric Delaître, Laurent Demagistri, Izildinha de Souza Miranda, Thérèse Libourel Rouge, Michel Petit To cite this version: Alessio Moreira dos Santos, Danielle Mitja, Eric Delaître, Laurent Demagistri, Izildinha de Souza Miranda, et al.. Estimating babassu palm density using automatic palm tree detection with very high spatial resolution satellite images. Journal of Environmental Management, Elsevier, 2017, 193, pp.40 - 51. 10.1016/j.jenvman.2017.02.004. ird-01715147 HAL Id: ird-01715147 https://hal.ird.fr/ird-01715147 Submitted on 22 Feb 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 Estimating babassu palm density using automatic palm tree detection with very high 2 spatial resolution satellite images 3 Alessio Moreira dos Santos ab*, Danielle Mitjac, Eric Delaîtrec, Laurent Demagistric, Izildinha de 4 Souza Mirandaa, Thérèse Libourelc, Michel Petitd. 5 6 a. Universidade Federal Rural da Amazonia (UFRA), CP.917, Belém, Pará, 66077-530, Belém, 7 Brazil. [email protected], [email protected] 8 b. Universidade Federal do Sul e Sudeste do Pará (UNIFESSPA), Folha 31, Quadra 07, Lote 9 Especial, Nova Marabá, 68507-590, Marabá, Brazil. -

Coconut Genetic Resources
Coconut Genetic Resources Pons Batugal, V. Ramanatha Rao and Jeffrey Oliver, editors i COCONUT GENETIC RESOURCES The International Plant Genetic Resources Institute (IPGRI) is an independent international scientific organization that seeks to improve the well-being of present and future generations of people by enhancing conservation and the deployment of agricultural biodiversity on farms and in forests. It is one of 15 Future Harvest Centres supported by the Consultative Group on International Agricultural Research (CGIAR), an association of public and private members who support efforts to mobilize cutting-edge science to reduce hunger and poverty, improve human nutrition and health, and protect the environment. IPGRI has its headquarters in Maccarese, near Rome, Italy, with offices in more than 20 other countries worldwide. The Institute operates through four programmes: Diversity for Livelihoods, Understanding and Managing Biodiversity, Global Partnerships, and Improving Livelihoods in Commodity-based Systems. The international status of IPGRI is conferred under an Establishment Agreement which, by January 2005, had been signed by the Governments of Algeria, Australia, Belgium, Benin, Bolivia, Brazil, Burkina Faso, Cameroon, Chile, China, Congo, Costa Rica, Côte d’Ivoire, Cyprus, Czech Republic, Denmark, Ecuador, Egypt, Greece, Guinea, Hungary, India, Indonesia, Iran, Israel, Italy, Jordan, Kenya, Malaysia, Mauritania, Morocco, Norway, Pakistan, Panama, Peru, Poland, Portugal, Romania, Russia, Senegal, Slovakia, Sudan, Switzerland, Syria, Tunisia, Turkey, Uganda and Ukraine. Financial support for IPGRI’s research is provided by more than 150 donors, including governments, private foundations and international organizations. For details of donors and research activities please see IPGRI’s Annual Reports, which are available in printed form on request from [email protected] or from IPGRI’s Web site (www.ipgri.cgiar.org). -

Astrocaryum Chambira)
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/279205063 Chambira o cumare (Astrocaryum chambira) Chapter · January 2013 CITATIONS READS 0 1,427 1 author: Néstor García Pontificia Universidad Javeriana 42 PUBLICATIONS 236 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Investigación e innovación tecnológica y apropiación social de conocimiento científico de orquídeas nativas de Cundinamarca View project Demografía, manejo y conservación de Attalea nucifera (Arecaceae) en la cuenca del río Magdalena View project All content following this page was uploaded by Néstor García on 28 July 2015. The user has requested enhancement of the downloaded file. Cosechar sin destruir Aprovechamiento sostenible de palmas colombianas Rodrigo Bernal y Gloria Galeano Editores Bogotá, D. C., Colombia, octubre de 2013 Catalogación en la publicación Universidad Nacional de Colombia Cosechar sin destruir : aprovechamiento sostenible de palmas colombianas / editores Rodrigo Bernal y Gloria Galeano. -- Bogotá : Universidad Nacional de Colombia. Facultad de Ciencias. Instituto de Ciencias Naturales : PALMS : Colciencias, 2013 244 páginas : ilustraciones Incluye referencias bibliográficas ISBN : 978-958-761-611-8 1. Palmas – Colombia 2. Ecología de cultivos – Colombia 3. Industria de la palma - Tecnología poscosecha 4. Palmas - Distribución geográfica – Colombia 5. Silvicultura sostenible – Colombia 6. Etnobotánica – Colombia I. Bernal González,