Radioactive Decay Summaries of Concepts Pertaining to Radiation and Radioactivity, Including: an Atom Is the Smallest Particle of an Element
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Standard Conversion Factors
Standard conversion factors 7 1 tonne of oil equivalent (toe) = 10 kilocalories = 396.83 therms = 41.868 GJ = 11,630 kWh 1 therm = 100,000 British thermal units (Btu) The following prefixes are used for multiples of joules, watts and watt hours: kilo (k) = 1,000 or 103 mega (M) = 1,000,000 or 106 giga (G) = 1,000,000,000 or 109 tera (T) = 1,000,000,000,000 or 1012 peta (P) = 1,000,000,000,000,000 or 1015 WEIGHT 1 kilogramme (kg) = 2.2046 pounds (lb) VOLUME 1 cubic metre (cu m) = 35.31 cu ft 1 pound (lb) = 0.4536 kg 1 cubic foot (cu ft) = 0.02832 cu m 1 tonne (t) = 1,000 kg 1 litre = 0.22 Imperial = 0.9842 long ton gallon (UK gal.) = 1.102 short ton (sh tn) 1 UK gallon = 8 UK pints 1 Statute or long ton = 2,240 lb = 1.201 U.S. gallons (US gal) = 1.016 t = 4.54609 litres = 1.120 sh tn 1 barrel = 159.0 litres = 34.97 UK gal = 42 US gal LENGTH 1 mile = 1.6093 kilometres 1 kilometre (km) = 0.62137 miles TEMPERATURE 1 scale degree Celsius (C) = 1.8 scale degrees Fahrenheit (F) For conversion of temperatures: °C = 5/9 (°F - 32); °F = 9/5 °C + 32 Average conversion factors for petroleum Imperial Litres Imperial Litres gallons per gallons per per tonne tonne per tonne tonne Crude oil: Gas/diesel oil: Indigenous Gas oil 257 1,167 Imported Marine diesel oil 253 1,150 Average of refining throughput Fuel oil: Ethane All grades 222 1,021 Propane Light fuel oil: Butane 1% or less sulphur 1,071 Naphtha (l.d.f.) >1% sulphur 232 1,071 Medium fuel oil: Aviation gasoline 1% or less sulphur 237 1,079 >1% sulphur 1,028 Motor -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Look Back at 2019 (PDF, 1.14
A look back at c&a look back 2019 2 019 Recent years have seen a combination of natural disasters, extreme weather and political chaos and 2019 has been more of the same! Cyclone hits Mozambique More than 1,000 people lost Airlines Flight 302 crashed with 157 people onboard. Five years after their lives when cyclones hit Prince Harry and Meghan, Duke and New Zealand Mozambique, Zimbabwe and Malaysia Airlines Flight 17 was shot Duchess of Sussex. Al Noor down over Ukraine killing 298, three Malawi. A dam collapse at The crane, access and telehandler Mosque Russians and a Ukrainian have been shooting the Córrego do Feijão iron ore markets generally remained busy charged. mine in Brazil killed 237 with although the continued economic 33 missing, while horrendous President Trump continued be a and political uncertainty began to rainfall in Europe left dozens of disruptive force on the world stage, choke some capital investment people losing their lives across using tariffs as a diplomatic weapon, leading to shrinking order books as Austria, Czech Republic, Germany, raising them against products the year end approached. Hungary and Slovakia, Spain, of almost every trade partner, The following is a reminder France and Italy. Northern England from steel to Scotch Whisky, as of some of the key stories we experienced the second or third he escalated his trade war with carried in the magazine this year. ‘Once in a hundred year’ flooding China and the EU. He also pulled in little more than 10 years. the US out of the long standing Hong Kong Terrorism was largely confined to Intermediate-Range Nuclear Forces demonstrations the Middle East and parts of Africa, Treaty, withdrawing support for but in New Zealand 50 people died Kurdish allies, and stating that and 50 were wounded when a Israel’s occupation of Palestine’s gunman opened fire at the Al Noor West Bank was legal. -

Conversion of Units
CCoonnvveerrssiioonn ooff UUnniittss1 Length 1 millimeter = 0.0394 inch 1 centimeter = 0.394 inch 1 meter = 39.4 inches 1 meter = 3.2808 feet 1 kilometer = 0.62137 mile 1 inch = 25.4 millimeters 1 inch = 2.54 centimeters 1 foot = 30.48 centimeters 1 foot = 0.305 meter 1 yard = 0.9144 meter 1 mile = 1.60935 meters Area 1 square millimeter = 0.00155 square inch 1 square centimeter = 0.1550 square inch 1 square meter = 10.764 square feet 1 square kilometer = 0.3861 square mile 1 square inch = 6.452 square centimeters 1 square inch = 645.2 square millimeters 1 square foot = 0.0929 square meter 1 square yard = 0.8361 square meter 1 square mile = 2.590 square kilometers Volume 1 cubic millimeter = 0.0006 cubic inch 1 cubic centimeter = 0.0610 cubic inch 1 liter = 0.26418 US gallon 1 liter = 1000 cubic centimeters 1 cubic meter = 1.3079 cubic yards 1 cubic meter = 35.313 cubic feet 1 cubic meter = 264.143 US gallons 1 cubic inch = 16.3872 cubic centimeters 1 cubic inch = 1638.72 cubic millimeters 1 US gallon = 3.78533 liters 1 cubic foot = 28.32 liters 1 cubic foot = 0.02832 cubic meters 1 cubic foot = 1728 cubic inches 1 cubic foot = 7.48 US gallons 1 cubic yard = 0.7656 cubic meter 1 Source: North American Combustion Handbook, Volume II, North American Mfg. Co., Cleveland OH 44105 USA, p. 317 - 325. x CCoonnvveerrssiioonn ooff UUnniittss ((ccoonnttiinnuueedd)) Weight 1 gram = 15.4324 grains 1 kilogram = 2.20462 pounds 1 Tonne (metric) = 1000 kilograms 1 Tonne (metric) = 2200 pounds 1 Tonne (metric) = 1.1023 US Tons 1 grain = 0.0648 ounce 1 ounce = 28.3495 grams 1 pound = 0.45359 kilogram 1 pound = 16 ounces 1 Ton (US) = 2000 pounds 1 Ton (US) = 907.18 kilograms Pressure 1 kg/cm 2 = 14.22 psi (lb/in 2) 1 bar = 750.1 mm Mercury 1 bar = 100.0 kPa (kilopascal) 1 bar = 10,200 mm WC 1 bar = 14.50 psi (lb/in 2) 1 psi (lb/in 2) = 0.0703 kg/cm 2 1 psi (lb/in 2) = 0.06897 bar Power, Work, and Heat 1 Calorie = 0.00397 Btu 1 Kilocalorie = 3.968 Btu 1 KCalorie/Kg = 1.8037 Btu/lb 1 Watt = 0.056884 BTU/min. -

ENERGY NUMERACY: UNITS Robert A
ENERGY NUMERACY: UNITS Robert A. James Pillsbury Winthrop Shaw Pittman LLP ENERGY UNITS dimensions, DIM: Mass, Length, Time; units: SI Système Internationale, United States customary 2 Mass DIM M Force F = ma, DIM ML/T Work or Energy (Capacity) 2 2 2 2 SI kilogram, kg = (10cm)3 water ≈ 2.2 lbs. SI newton, N = kg-m/sec through a DIM ML/T x L = ML /T accelerate 2 1 metric tonne, m.t. = 1000 kg also Weight; Earth’s a is g ≈ -9.8m/sec distance SI joule, J = newton-meter, kg-m2/sec2 1 pound (“libra”), lb. ≈ 4.45 N SI 1 food Calorie ≈ 4184 J SI PER each time UNIT 1 short ton, s.t. = 2000 lbs.≈ 907 N SI 1 British thermal unit, Btu ≈ 1055 J SI Kinetic = mv2/2; Potential = mgh Power (Rate) DIM ML2/T2 ÷ T = ML2/T3 SI watt, W = J/sec = kg-m2/sec3 1 horsepower ≈ 746 W SI FOSSIL FUEL MEASURES Energy (Output) DIM ML2/T3 x T = ML2/T2 again↑ SI joule again, but it is a tiny unit FOR a time PERIOD 1 barrel of oil (“blue barrel”), bbl = 42 U.S. gallons (≈158.76 liters SI) 1 watt-hour, Wh = 3600 J ≈ 3.412 Btu 1000 standard cubic feet of gas, Mcf ≈ 1 million Btu or MMBtu (≈28.3 m3 SI) 1 kilowatt-hour, kWh ≈ 3412 Btu 1 barrel/day, bpd ≈ 50 m.t./year SI 1 gigajoule, GJ ≈.947 MMBtu ≈ .278 MWh 1000 bpd ≈ 2 trillion Btu per year 1 MMBtu ≈ 1.055 GJ ≈ .293 MWh 1 m.t. -

General Conversion Table in Alphabetical Order
General Conversion Table In Alphabetical Order UNIT x FACTOR = UNIT UNIT x FACTOR = UNIT Acceleration gravity 9.80665 meter/second2 british thermal unit (BTU) 1054.35 watt-seconds Acceleration gravity 32.2 feet/second2 british thermal unit (BTU) 10.544 x 103 ergs Acceleration gravity 9.80665 meter/second2 british thermal unit (BTU) 0.999331 BTU (IST) Acceleration gravity 32.2 feet/second2 BTU/min 0.01758 kilowatts acre 4,046.856 meter2 BTU/min 0.02358 horsepower acre 0.40469 hectare byte 8.000001 bits acre 43,560.0 foot2 calorie, g 0.00397 british thermal unit acre 4,840.0 yard2 calorie, g 0.00116 watt-hour acre 0.00156 mile2 (statute) calorie, g 4184.00 x 103 ergs acre 0.00404686 kilometer2 calorie, g 3.08596 foot pound-force acre 160 rods2 calorie, g 4.184 joules acre feet 1,233.489 meter2 calorie, g 0.000001162 kilowatt-hour acre feet 325,851.0 gallon (US) calorie, g 42664.9 gram-force cm acre feet 1,233.489 meter3 calorie, g/hr 0.00397 btu/hr acre feet 325,851.0 gallon calorie, g/hr 0.0697 watts acre-feet 43560 feet3 candle/cm2 12.566 candle/inch2 acre-feet 102.7901531 meter3 candle/cm2 10000.0 candle/meter2 acre-feet 134.44 yards3 candle/inch2 144.0 candle/foot2 ampere 1 coulombs/second candle power 12.566 lumens ampere 0.0000103638 faradays/second carats 3.0865 grains ampere 2997930000.0 statamperes carats 200.0 milligrams ampere 1000 milliamperes celsius 1.8C°+ 32 fahrenheit ampere/meter 3600 coulombs celsius 273.16 + C° kelvin angstrom 0.0001 microns centimeter 0.39370 inch angstrom 0.1 millimicrons centimeter 0.03281 foot atmosphere -

Annexes A.1 A
World Energy Council 2013 World Energy Resources: Annexes A.1 A Annexes Contents 1. Abbreviations and Acronyms / page 2 2. Conversion Factors and Energy Equivalents / page 5 3. Definitions / page 6 A.2 World Energy Resources: Annexes World Energy Council 2013 1. Abbreviations and Acronyms 103 kilo (k) CMM coal mine methane 106 mega (M) CNG compressed natural gas 9 10 giga (G) CO2e carbon dioxide equivalent 1012 tera (T) COP3 Conference of the Parties III, Kyoto1997 1015 peta (P) cP centipoise 1018 exa (E) CSP centralised solar power 1021 zetta (Z) d day ABWR advanced boiling water reactor DC direct current AC alternating current DHW domestic hot water AHWR advanced heavy water reactor DOWA deep ocean water applications API American Petroleum Institute ECE Economic Commission for Europe APR advanced pressurised reactor EIA U.S. Energy Information Administration / environmental impact assessment APWR advanced pressurised water reactor EOR enhanced oil recovery b/d barrels per day EPIA European Photovoltaic Industry Association bbl barrel EPR European pressurised water reactor bcf billion cubic feet ESTIF European Solar Thermal Industry Federation bcm billion cubic metres ETBE ethyl tertiary butyl ether BGR Bundesanstalt für Geowissenschaften und Rohstoffe F Fahrenheit billion 109 FAO UN Food and Agriculture Organization BIPV building integrated PV FBR fast breeder reactor BNPP buoyant nuclear power plant FID final investment decision boe barrel of oil equivalent FSU former Soviet Union BOO build, own, operate ft feet BOT build, operate, -

Comparing Energy and Heat Units !! (Intro to Dimensional Analysis)
Module 1, Lesson 2 Comparing Energy and Heat Units !! (Intro to Dimensional Analysis) Objective: By the end of this lesson you will be able to convert between the various units of heat energy commonly used, and make meaningful comparisons between energy usage. You will find that many things become apparent when appropriate units are used. You will also become familiar with the idea of dimensional analysis, one of the most powerful tools in a physicists toolkit. 1. Energy Cost Comparison 1.1 Apples and Oranges In Vancouver BC, energy to power our lives comes in three major forms: natural gas to heat our home, electricity to light it and run appliances (mostly from hydro-electric dams), and oil in the form of the gasoline that we put in out cars. Natural gas is sold by the GJ, electricity by the kWh, and gasoline by the litre. The unit costs we pay in Vancouver are, approximately (as of June 2010, in $CDN, which today is almost the same as the $US): Natural gas: $15/GJ Electricity: $0.06/kWh Gasoline: $1.15/L These are all sources of energy, but we use different units to measure our consumption of them. As they are now comparing them is comparing apples to oranges. Let's compare apples with apples and compare the costs in terms of $/J and $/tonne CO2. Natural gas is already sold in units of dollars per joule, so we will look at electricity first. Electricity is solve in units of kilowatt hours (kWh). Kilowatt hours are a strange unit. -
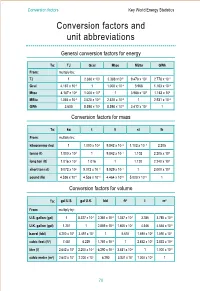
Conversion Factors and Unit Abbreviations
Conversion factors Key World Energy Statistics Conversion factors and unit abbreviations General conversion factors for energy To: TJ Gcal Mtoe MBtu GWh From: multiply by: TJ 1 2.388 x 102 2.388 x10-5 9.478 x 102 2.778 x 10-1 Gcal 4.187 x 10-3 1 1.000 x 10-7 3.968 1.163 x 10-3 Mtoe 4.187 x 104 1.000 x 107 1 3.968 x 107 1.163 x 104 MBtu 1.055 x 10-3 2.520 x 10-1 2.520 x 10-8 1 2.931 x 10-4 GWh 3.600 8.598 x 102 8.598 x 10-5 3.412 x 103 1 Conversion factors for mass To: kg t lt st lb From: multiply by: kilogramme (kg) 1 1.000 x 10-3 9.842 x 10–4 1.102 x 10–3 2.205 tonne (t) 1.000 x 103 1 9.842 x 10–1 1.102 2.205 x 103 long ton (lt) 1.016 x 103 1.016 1 1.120 2.240 x 103 short ton (st) 9.072 x 102 9.072 x 10–1 8.929 x 10–1 1 2.000 x 103 pound (lb) 4.536 x 10–1 4.536 x 10–4 4.464 x 10–4 5.000 x 10–4 1 Conversion factors for volume To: gal U.S. gal U.K. bbl ft3 l m3 From: multiply by: U.S. gallon (gal) 1 8.327 x 10-1 2.381 x 10-2 1.337 x 10-1 3.785 3.785 x 10-3 U.K. -

The International System of Units (SI) - Conversion Factors For
NIST Special Publication 1038 The International System of Units (SI) – Conversion Factors for General Use Kenneth Butcher Linda Crown Elizabeth J. Gentry Weights and Measures Division Technology Services NIST Special Publication 1038 The International System of Units (SI) - Conversion Factors for General Use Editors: Kenneth S. Butcher Linda D. Crown Elizabeth J. Gentry Weights and Measures Division Carol Hockert, Chief Weights and Measures Division Technology Services National Institute of Standards and Technology May 2006 U.S. Department of Commerce Carlo M. Gutierrez, Secretary Technology Administration Robert Cresanti, Under Secretary of Commerce for Technology National Institute of Standards and Technology William Jeffrey, Director Certain commercial entities, equipment, or materials may be identified in this document in order to describe an experimental procedure or concept adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the entities, materials, or equipment are necessarily the best available for the purpose. National Institute of Standards and Technology Special Publications 1038 Natl. Inst. Stand. Technol. Spec. Pub. 1038, 24 pages (May 2006) Available through NIST Weights and Measures Division STOP 2600 Gaithersburg, MD 20899-2600 Phone: (301) 975-4004 — Fax: (301) 926-0647 Internet: www.nist.gov/owm or www.nist.gov/metric TABLE OF CONTENTS FOREWORD.................................................................................................................................................................v -

The International System of Units (SI)
The International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 330 2008 Edition Barry N. Taylor and Ambler Thompson, Editors NIST SPECIAL PUBLICATION 330 2008 EDITION THE INTERNATIONAL SYSTEM OF UNITS (SI) Editors: Barry N. Taylor Physics Laboratory Ambler Thompson Technology Services National Institute of Standards and Technology Gaithersburg, MD 20899 United States version of the English text of the eighth edition (2006) of the International Bureau of Weights and Measures publication Le Système International d’ Unités (SI) (Supersedes NIST Special Publication 330, 2001 Edition) Issued March 2008 U.S. DEPARTMENT OF COMMERCE, Carlos M. Gutierrez, Secretary NATIONAL INSTITUTE OF STANDARDS AND TECHNOLOGY, James Turner, Acting Director National Institute of Standards and Technology Special Publication 330, 2008 Edition Natl. Inst. Stand. Technol. Spec. Pub. 330, 2008 Ed., 96 pages (March 2008) CODEN: NSPUE2 WASHINGTON 2008 Foreword The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant system used in science, the SI is rapidly becoming the dominant measurement system used in international commerce. In recognition of this fact and the increasing global nature of the marketplace, the Omnibus Trade and Competitiveness Act of 1988, which changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. industry increase its competitiveness, designates “the metric system of measurement as the preferred system of weights and measures for United States trade and commerce.” The definitive international reference on the SI is a booklet published by the International Bureau of Weights and Measures (BIPM, Bureau International des Poids et Mesures) and often referred to as the BIPM SI Brochure. -

Approximate Conversion Factors
Approximate conversion factors Statistical Review of World Energy Crude oil* To convert Tonnes (metric) Kilolitres Barrels US gallons Tonnes/year From Multiply by Tonnes (metric) 1 1.165 7.33 307.86 – Kilolitres 0.8581 1 6.2898 264.17 – Barrels 0.1364 0.159 1 42 – US gallons 0.00325 0.0038 0.0238 1 – Barrels/day – – – – 49.8 *Based on the worldwide average gravity Products To convert Barrels to Tonnes to Kilolitres to Tonnes to Tonnes to Tonnes to tonnes barrels tonnes kilolitres gigajoules barrels oil equivalent From Multiply by Ethane 0.059 16.850 0.373 2.679 49.400 8.073 Liquified petroleum gas (LPG) 0.086 11.600 0.541 1.849 46.150 7.542 Gasoline 0.120 8.350 0.753 1.328 44.750 7.313 Kerosene 0.127 7.880 0.798 1.253 43.920 7.177 Gas oil/diesel 0.134 7.460 0.843 1.186 43.380 7.089 Residual fuel oil 0.157 6.350 0.991 1.010 41.570 6.793 Product basket 0.124 8.058 0.781 1.281 43.076 7.039 Approximate conversion factors – Statistical Review of World Energy – updated July 2021 Page | 1 Natural gas and LNG To convert Billion cubic Billion cubic Petajoules Million Million Trillion Million metres NG feet NG NG tonnes oil tonnes LNG British barrels oil equivalent thermal equivalent units From Multiply by 1 billion cubic metres NG 1.000 35.315 36.000 0.860 0.735 34.121 5.883 1 billion cubic feet NG 0.028 1.000 1.019 0.024 0.021 0.966 0.167 1 petajoule NG 0.028 0.981 1.000 0.024 0.021 0.952 0.164 1 million tonnes oil equivalent 1.163 41.071 41.868 1.000 0.855 39.683 6.842 1 million tonnes LNG 1.360 48.028 48.747 1.169 1.000 46.405 8.001 1 trillion