COMPOSITION of HAWTHORN (Crataegus Spp.) FRUITS and LEAVES and EMBLIC LEAFFLOWER (Phyllanthus Emblica) FRUITS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CHANGES of POLYPHENOL COMPOUND CONCENTRATIONS in HYBRIDS of NANTE TYPE CARROTS DURING STORAGE Ingrîda Augðpole, Tatjana Kince, and Ingmârs Cinkmanis
PROCEEDINGS OF THE LATVIAN ACADEMY OF SCIENCES. Section B, Vol. 71 (2017), No. 6 (711), pp. 492–495. DOI: 10.1515/prolas-2017-0085 CHANGES OF POLYPHENOL COMPOUND CONCENTRATIONS IN HYBRIDS OF NANTE TYPE CARROTS DURING STORAGE Ingrîda Augðpole, Tatjana Kince, and Ingmârs Cinkmanis Faculty of Food Technology, Latvia University of Agriculture, 22 Rîgas Str., Jelgava, LV-3001, LATVIA Corresponding author, [email protected] Communicated by Andris Ozols The main purpose of the study was to determine changes of polyphenol concentrations in hybrids of Nante type carrots during storage. Fresh Nante type ‘Forto’ variety carrots and carrot hybrids ‘Bolero’ F1, ‘Champion’ F1, and ‘Maestro’ F1 were cultivated in the Zemgale region of Latvia. Car- rots were stored for six months in air (+3 ± 1 oC, RH = 89 ± 1%) and polyphenol compound con- centrations were determined at two month intervals. High-performance liquid chromatography was used to determine concentrations of eight polyphenols in carrots: gallic acid, catechin, epicatechin, caffeic acid, chlorogenic acid, ferulic acid, vanillin, and rutin. Significant differences occurred in polyphenol concentrations of fresh Nante type variety ‘Forto’ carrots and several hy- brids (‘Bolero’ F1, ‘Champion’ F1, and ‘Maestro’ F1) during storage. After six months of storage, the concentration of polyphenol compounds of Nante type carrots decreased — caffeic acid by 64.6%, chlorogenic acid — by 37.9% and vanillin — by 81.5%. However, during storage, concen- tration of some polyphenol compounds increased, as catechin by 30.5%, epicatechin by 85.2%, gallic acid by 48.5% and ferulic acid by 87.9%. Key words: carrots, polyphenols compounds, storage. INTRODUCTION rings to one another. -

Tannic Acid-Based Prepolymer Systems for Enhanced Intumescence in Epoxy Thermosets
Cite this article Themed Issue: Sustainable flame Keywords: environmental impact/green Korey M, Johnson A, Webb W et al. (2020) retarded materials polymers/sustainable materials Tannic acid-based prepolymer systems for enhanced intumescence in epoxy thermosets. Paper 1900061 Green Materials 8(3): 150–161, Received 29/09/2019; Accepted 05/03/2020 https://doi.org/10.1680/jgrma.19.00061 Published online 06/04/2020 ICE Publishing: All rights reserved Green Materials Tannic acid-based prepolymer systems for enhanced intumescence in epoxy thermosets Matthew Korey Mark Dietenberger Graduate Research Assistant, Purdue University, West Lafayette, IN, USA Research General Engineer, Forest Products Laboratory, Madison, WI, USA (Orcid:0000-0002-2285-5646) Jeffrey Youngblood Alexander Johnson Professor, Purdue University, West Lafayette, IN, USA Undergraduate Research Assistant, Purdue University, West Lafayette, IN, USA (Orcid:0000-0002-8720-8642) William Webb John Howarter Staff, Career Academy, San Diego, CA, USA Associate Professor, Purdue University, West Lafayette, IN, USA (corresponding author: [email protected]) Tannic acid (TA) is a bio-based high-molecular-weight organic molecule. Although biologically sourced, TA is a pollutant in industrial wastewater streams, and there is desire to find applications in which to downcycle this molecule. Many flame retardants (FRs) used in epoxy are synthesized from petroleum-based monomers. Various bio-based modifiers have been developed, but increasing the flame retardancy of the system without trade-offs with other properties has proved challenging. In this work, TA is incorporated into the thermoset. The molecular behavior of the system was dependent on the TA loading, with low concentrations causing the molecule to be surface-functionalized, while at higher concentrations the molecule was cross-linked into the network. -

Black Pearls
Number 22 The Journal of the AMERICAN BOTANI CAL COUNCU.. and the HERB RESEARCH FOUNDATION Hawthorn -A Literature Review Special Report: Black Pearls - Prescription Drugs Masquerade as Chinese Herbal Arthritis Formula FROM THE EDITOR In This Issue his issue of HerbalGram offers some good news and some herbal combination for use in rheumatoid arthritis and related bad news. First the good news. Our Legal and Regulatory inflammatory conditions, this product has been tested repeatedly Tsection is devoted to a recent clarification by the Canadian and shown positive for the presence of unlabeled prescription drugs. Health Protection Branch (Canada's counterpart to our FDA)of its Herb Research Foundation President Rob McCaleb and I have willingness to grant "Traditional Medicine" status to many medici spent several months researching the latest resurgence in sales of nal herb products sold in Canada under the already existing approval this and related products. We have made every attempt to follow up process for over-the-counter remedies. This announcement has on many avenues to determine whether these products contain un been hailed as a positive step by almost everyone with whom we labeled drugs, and whether or not they are being marketed fraudu have talked, both in academia and in the herb industry. lently. Herb marketers and consumers alike should be concerned More good news is found in the literature review on Hawthorn. whenever prescription drugs are presented for sale as "natural" and Steven Foster has joined Christopher Hobbs in preparing a compre "herbal." You will find our report on page 4. hensive view of a plant with a long history of use as both food and In addition, we present the usual array of interesting blurbs, medicine. -

The Use of Plants in the Traditional Management of Diabetes in Nigeria: Pharmacological and Toxicological Considerations
Journal of Ethnopharmacology 155 (2014) 857–924 Contents lists available at ScienceDirect Journal of Ethnopharmacology journal homepage: www.elsevier.com/locate/jep Review The use of plants in the traditional management of diabetes in Nigeria: Pharmacological and toxicological considerations Udoamaka F. Ezuruike n, Jose M. Prieto 1 Center for Pharmacognosy and Phytotherapy, Department of Pharmaceutical and Biological Chemistry, School of Pharmacy, University College London, 29-39 Brunswick Square, WC1N 1AX London, United Kingdom article info abstract Article history: Ethnopharmacological relevance: The prevalence of diabetes is on a steady increase worldwide and it is Received 15 November 2013 now identified as one of the main threats to human health in the 21st century. In Nigeria, the use of Received in revised form herbal medicine alone or alongside prescription drugs for its management is quite common. We hereby 26 May 2014 carry out a review of medicinal plants traditionally used for diabetes management in Nigeria. Based on Accepted 26 May 2014 the available evidence on the species' pharmacology and safety, we highlight ways in which their Available online 12 June 2014 therapeutic potential can be properly harnessed for possible integration into the country's healthcare Keywords: system. Diabetes Materials and methods: Ethnobotanical information was obtained from a literature search of electronic Nigeria databases such as Google Scholar, Pubmed and Scopus up to 2013 for publications on medicinal plants Ethnopharmacology used in diabetes management, in which the place of use and/or sample collection was identified as Herb–drug interactions Nigeria. ‘Diabetes’ and ‘Nigeria’ were used as keywords for the primary searches; and then ‘Plant name – WHO Traditional Medicine Strategy accepted or synonyms’, ‘Constituents’, ‘Drug interaction’ and/or ‘Toxicity’ for the secondary searches. -

ECOLOGICAL and ECONOMIC IMPORTANCE of STUDYING PROPAGATION TECHNIQUES of COMMON HAWTHORN Crataegus Monogyna Jacq. G
СИБИРСКИЙ ЛЕСНОЙ ЖУРНАЛ. 2019. № 4. С. 63–67 UDC 581.16/581.6/581.9:634.17 ECOLOGICAL AND ECONOMIC IMPORTANCE OF STUDYING PROPAGATION TECHNIQUES OF COMMON HAWTHORN Crataegus monogyna Jacq. G. Özyurt, Z. Yücesan, N. Ak, E. Oktan, A. Ö. Üçler Karadeniz Technical University Trabzon, 61080 Turkey E-mail: [email protected], [email protected], [email protected], [email protected], [email protected] Received 11.04.2019 Climate change as a fact of global warming requires the development of different perspectives on the planning and implementation of sustainable forestry techniques. Increasing temperatures cause drought on a global basis. In connection with, this using drought tolerant species in afforestation work is of great importance. In recent years Crataegus L. species (hawthorn) are also involved in afforestation. One of these species, C. monogyna, is characterized by drought tolerance. Furthermore, C. monogyna is the most important nonwood forest product species of Turkey. Hawthorn is widely used in medicine (treatment of coronary heart diseases), and cosmetics industry, agriculture and animal husbandry and human nutrition. On the other hand, it is used in erosion control, afforestation, industrial energy resources and for landscaping. Economic and ecological contribution of hawthorn to the national economy is quite high. Therefore, determination of suitable generative and vegetative reproduction techniques and vast production of seedlings of hawthorn species are extremely important. The characteristics of generative and vegetative propagation of Crataegus are discussed. For generative propagation of hawthorn species, the most effective and suitable procedure is treatment of seeds in ash solution. For vegetative propagation in culture in vitro the growth induced by BA (benzyladenine) and IBA (indole butyric acid) hormones increases the rate of callus formation and rooting. -

Chemistry and Pharmacology of Kinkéliba (Combretum
CHEMISTRY AND PHARMACOLOGY OF KINKÉLIBA (COMBRETUM MICRANTHUM), A WEST AFRICAN MEDICINAL PLANT By CARA RENAE WELCH A Dissertation submitted to the Graduate School-New Brunswick Rutgers, The State University of New Jersey in partial fulfillment of the requirements for the degree of Doctor of Philosophy Graduate Program in Medicinal Chemistry written under the direction of Dr. James E. Simon and approved by ______________________________ ______________________________ ______________________________ ______________________________ New Brunswick, New Jersey January, 2010 ABSTRACT OF THE DISSERTATION Chemistry and Pharmacology of Kinkéliba (Combretum micranthum), a West African Medicinal Plant by CARA RENAE WELCH Dissertation Director: James E. Simon Kinkéliba (Combretum micranthum, Fam. Combretaceae) is an undomesticated shrub species of western Africa and is one of the most popular traditional bush teas of Senegal. The herbal beverage is traditionally used for weight loss, digestion, as a diuretic and mild antibiotic, and to relieve pain. The fresh leaves are used to treat malarial fever. Leaf extracts, the most biologically active plant tissue relative to stem, bark and roots, were screened for antioxidant capacity, measuring the removal of a radical by UV/VIS spectrophotometry, anti-inflammatory activity, measuring inducible nitric oxide synthase (iNOS) in RAW 264.7 macrophage cells, and glucose-lowering activity, measuring phosphoenolpyruvate carboxykinase (PEPCK) mRNA expression in an H4IIE rat hepatoma cell line. Radical oxygen scavenging activity, or antioxidant capacity, was utilized for initially directing the fractionation; highlighted subfractions and isolated compounds were subsequently tested for anti-inflammatory and glucose-lowering activities. The ethyl acetate and n-butanol fractions of the crude leaf extract were fractionated leading to the isolation and identification of a number of polyphenolic ii compounds. -
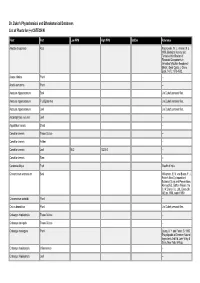
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Plants for (+)-CATECHIN
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Plants for (+)-CATECHIN Plant Part Low PPM High PPM StdDev Reference Abutilon theophrasti Root Paszkowski, W. L., Kremer, R. J. 1988. Biological Activity and Tentative Identification of Flavonoid Components in Velvetleaf (Abutilon theophrasti Medik.) Seed Coats. J. Chem. Ecol., 14(7): 1573-1582. Acacia nilotica Plant -- Acacia decurrens Plant -- Aesculus hippocastanum Bark Jim Duke's personal files. Aesculus hippocastanum Fruit Epidermis Jim Duke's personal files. Aesculus hippocastanum Leaf Jim Duke's personal files. Arctostaphylos uva-ursi Leaf -- Aspalathus linearis Shoot -- Camellia sinensis Tissue Culture -- Camellia sinensis Anther -- Camellia sinensis Leaf 85.2 13200.0 -- Camellia sinensis Stem -- Ceratonia siliqua Fruit Wealth of India. Cinnamomum aromaticum Bark Williamson, E. M. and Evans, F. J., Potter's New Cyclopaedia of Botanical Drugs and Preparations, Revised Ed., Saffron Walden, the C. W. Daniel Co., Ltd., Essex UK, 362 pp, 1988, reprint 1989. Cinnamomum sieboldii Plant -- Cnicus benedictus Plant Jim Duke's personal files. Crataegus rhipidophylla Tissue Culture -- Crataegus laevigata Tissue Culture -- Crataegus monogyna Plant Leung, A. Y. and Foster, S. 1995. Encyclopedia of Common Natural Ingredients 2nd Ed. John Wiley & Sons, New York. 649 pp. Crataegus rhipidophylla Inflorescence -- Crataegus rhipidophylla Leaf -- Plant Part Low PPM High PPM StdDev Reference Crataegus laevigata Leaf -- Crataegus laevigata Plant Leung, A. Y. and Foster, S. 1995. Encyclopedia of Common Natural Ingredients 2nd Ed. John Wiley & Sons, New York. 649 pp. Crataegus rhipidophylla Bud -- Crataegus rhipidophylla Flower -- Crataegus laevigata Bud -- Crataegus laevigata Flower -- Croton lechleri Plant -- Eucalyptus globulus Leaf -- Fagopyrum esculentum Root -- Fagopyrum esculentum Tissue Culture -- Fallopia japonica Root 2.0 -- Geranium thunbergii Tissue Culture -- Ginkgo biloba Tissue Culture Jim Duke's personal files. -

World Journal of Pharmaceutical Research Vangalapati Et Al
World Journal of Pharmaceutical ReseaRch Vangalapati et al. World Journal of Pharmaceutical SJIF Research Impact Factor 5.045 Volume 3, Issue 6, 2127-2139. Review Article ISSN 2277 – 7105 A REVIEW ON CHEBULINIC ACID FROM MEDICINAL HERBS Surya Prakash DV1 and Meena Vangalapati2* 1 Research scholar, Centre for Biotechnology, Department of Chemical Engineering, Andhra University, Visakhapatnam-530003, Andhra Pradesh, India. 2* Associate Professor, Centre for Biotechnology, Department of Chemical Engineering, Andhra University, Visakhapatnam-530003, Andhra Pradesh, India. Article Received on ABSTRACT 30 June 2014, Chebulinic acid is a phenolic compound found in the fruits of Revised on 25 July 2014, Accepted on 20 August 2014 Terminalia chebula (Haritaki), Phyllanthus emblica (Amla) and seeds of Dimocarpus longan (Longan) species etc. It’s also known as 1,3,6- *Correspondence for Tri-O-galloyl-2,4-chebuloyl-β-D-glucopyranoside. Chebulinic acid is Author extracted from the medicinal herbs using soxhlet extractor, column Dr. Meena Vangalapati chromatography and high pressure liquid chromatography (HPLC) etc. Associate Professor, Centre for It showed many pharmacological activities including inhibition of Biotechnology, Department of cancer cell growth like human leukemia K562 cells, colon Chemical Engineering, Andhra University, adenocarcinoma HT-29 cell lines, anti-neisseria gonorrhoeae activity, Visakhapatnam-530003, inhibiting the contractile responses of cardiovascular muscles activities Andhra Pradesh, India. etc. KEY WORDS: Chebulinic acid, Terminalia chebula, Dimocarpus longan, Phyllanthus emblica, Anticancer activity, HPLC, Astringency. INTRODUCTION Chebulinic acid is a phenolic compound [1,2] commonly found in the fruits of Terminalia chebula, leaves and fruits of Phyllanthus emblica and seeds of Dimocarpus longan species, which has many potential uses in medicine. -

Are Supplements Supplemented? Evaluating the Composition of Complementary and Alternative Medicines Using Mass Spectrometry and Metabolomics
Are supplements supplemented? Evaluating the composition of complementary and alternative medicines using mass spectrometry and metabolomics By Elly Gwyn Crighton BForensics in Forensic Biology & Toxicology (First Class Honours) BSc in Molecular Biology & Biomedical Science This thesis is presented for the degree of Doctor of Philosophy Perth, Western Australia at Murdoch University 2020 Declaration I declare that: i. The thesis is my own account of my research, except where other sources are acknowledged. ii. The extent to which the work of others has been used is clearly stated in each chapter and certified by my supervisors. iii. The thesis contains as its main content, work that has not been previously submitted for a degree at any other university. i Abstract The complementary and alternative medicines (CAM) industry is worth over US$110 billion globally. Products are available to consumers with little medical advice; with many assuming that such products are ‘natural’ and therefore safe. However, with adulterated, contaminated and fraudulent products reported on overseas markets, consumers may be placing their health at risk. Previous studies into product content have reported undeclared plant materials, ingredient substitution, adulteration and contamination. However, no large-scale, independent audit of CAM has been undertaken to demonstrate these problems in Australia. This study aimed to investigate the content and quality of CAM products on the Australian market. 135 products were analysed using a combination of next-generation DNA sequencing and liquid chromatography-mass spectrometry. Nearly 50% of products tested had contamination issues, in terms of DNA, chemical composition or both. 5% of the samples contained undeclared pharmaceuticals. -

Molecular Docking Studies of Medicinal Compounds Against Aldose Reductase Drug Target for Treatment of Type 2 Diabetes Pratistha Singh*1, Preeti Yadav2, V.K
Intl. J. Bioinformatics and Biological Sci.: (V. 5 n.2, p. 91-116): December 2017 ©2017 New Delhi Publishers. All rights reserved DOI: 10.5958/2321-7111.2017.00012.9 Molecular Docking Studies of Medicinal Compounds against Aldose reductase Drug Target for Treatment of Type 2 Diabetes Pratistha Singh*1, Preeti Yadav2, V.K. Singh3 and A.K. Singh1 1Department of Dravyaguna, Faculty of Ayurveda, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India 2School of Biotechnology, Institute of Science, Banaras Hindu University, Varanasi, India 3Centre for Bioinformatics, School of Biotechnology, Institute of Science, Banaras Hindu University, Varanasi, India *Corresponding author: [email protected] ABSTRACT Type 2 Diabetes is a disease that manifests from combined effect of genetic and environmental stress on multiple tissues over a period of time. An enzyme, Aldose Reductase play an important role in oxidative stress and Diabetic Mellitus was selected as a target protein for in silico screening of suitable herbal inhibitors using molecular docking. In the present work best screened ligands Ajoene, 3-O-methyl-D-chiro-inositol (D-pinitol), Butein, Leucopelargonidin, Nimbidinin, Tolbutamide and Coumarin were used for docking calculation and isolated from Allium sativum, Glycine max, Butea monosperma, Thepsia populena, Ficus benghalensis, Azardirachta indica, Nelumbo nucifera, Aegle marmelos respectively. Herbacetin and Quercetin from Thepsia populena. The residues Gly18, Thr19, Trp20, Lys21, Asp43,Val47, Tyr48, Gln49, Asn50, Lys77, His110, Trp111, Thr113, Ser159, Asn160, Asn162, His163, Gln183, Tyr209, Ser210, Pro211, Leu212, Gly213, Ser214, Pro215, Asp216, Ala245, Ile260, Val264, Thr265, Arg268, Glu261, Asn262, Cys298, Ala299 and Leu300 were found conserved with binding site 1, which is major active site involved in interaction. -

Crataegus Laevigata 'Crimson Cloud' 'Crimson Cloud' English Hawthorn
Fact Sheet ST-211 November 1993 Crataegus laevigata ‘Crimson Cloud’ ‘Crimson Cloud’ English Hawthorn1 Edward F. Gilman and Dennis G. Watson2 INTRODUCTION Crimson Cloud (also known as ‘Superba’) English Hawthorn grows rapidly in a pyramidal form to about 20 feet, then the crown expands to become oval or irregular (Fig. 1). The tree tolerates most soils, growing well in clay, but prefers heavy, dry loam. The main ornamental feature is white and red flowers borne in spring which together give the tree a deep pink color. Fruits are red and quite showy but do not cover the tree. Though quite ornamental, Hawthorns are susceptible to insect and disease problems. Branching habit is decidedly drooping and care should be given when locating this tree near pedestrian or vehicular traffic. GENERAL INFORMATION Figure 1. Middle-aged ‘Crimson Cloud’ English Hawthorn. Scientific name: Crataegus laevigata ‘Crimson Cloud’ Availability: grown in small quantities by a small Pronunciation: kruh-TEE-gus lee-vih-GAY-tuh number of nurseries Common name(s): ‘Crimson Cloud’ English Hawthorn DESCRIPTION Family: Rosaceae USDA hardiness zones: 4B through 8 (Fig. 2) Height: 20 to 25 feet Origin: not native to North America Spread: 15 to 25 feet Uses: Bonsai; espalier; wide tree lawns (>6 feet Crown uniformity: irregular outline or silhouette wide); medium-sized tree lawns (4-6 feet wide); Crown shape: oval; pyramidal recommended for buffer strips around parking lots or Crown density: moderate for median strip plantings in the highway; reclamation Growth rate: medium plant; screen; narrow tree lawns (3-4 feet wide); Texture: fine specimen; sidewalk cutout (tree pit); residential street tree; tree has been successfully grown in urban areas where air pollution, poor drainage, compacted soil, and/or drought are common 1. -

In Vitro Bioaccessibility, Human Gut Microbiota Metabolites and Hepatoprotective Potential of Chebulic Ellagitannins: a Case of Padma Hepatenr Formulation
Article In Vitro Bioaccessibility, Human Gut Microbiota Metabolites and Hepatoprotective Potential of Chebulic Ellagitannins: A Case of Padma Hepatenr Formulation Daniil N. Olennikov 1,*, Nina I. Kashchenko 1,: and Nadezhda K. Chirikova 2,: Received: 28 August 2015 ; Accepted: 30 September 2015 ; Published: 13 October 2015 1 Laboratory of Medical and Biological Research, Institute of General and Experimental Biology, Siberian Division, Russian Academy of Science, Sakh’yanovoy Street 6, Ulan-Ude 670-047, Russia; [email protected] 2 Department of Biochemistry and Biotechnology, North-Eastern Federal University, 58 Belinsky Street, Yakutsk 677-027, Russian; [email protected] * Correspondence: [email protected]; Tel.: +7-9021-600-627; Fax: +7-3012-434-243 : These authors contributed equally to this work. Abstract: Chebulic ellagitannins (ChET) are plant-derived polyphenols containing chebulic acid subunits, possessing a wide spectrum of biological activities that might contribute to health benefits in humans. The herbal formulation Padma Hepaten containing ChETs as the main phenolics, is used as a hepatoprotective remedy. In the present study, an in vitro dynamic model simulating gastrointestinal digestion, including dialysability, was applied to estimate the bioaccessibility of the main phenolics of Padma Hepaten. Results indicated that phenolic release was mainly achieved during the gastric phase (recovery 59.38%–97.04%), with a slight further release during intestinal digestion. Dialysis experiments showed that dialysable phenolics were 64.11% and 22.93%–26.05% of their native concentrations, respectively, for gallic acid/simple gallate esters and ellagitanins/ellagic acid, in contrast to 20.67% and 28.37%–55.35% for the same groups in the non-dialyzed part of the intestinal media.