Unit (9) Carboxylic Acids, Esters, Amines, and Amides
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 21 the Chemistry of Carboxylic Acid Derivatives
Instructor Supplemental Solutions to Problems © 2010 Roberts and Company Publishers Chapter 21 The Chemistry of Carboxylic Acid Derivatives Solutions to In-Text Problems 21.1 (b) (d) (e) (h) 21.2 (a) butanenitrile (common: butyronitrile) (c) isopentyl 3-methylbutanoate (common: isoamyl isovalerate) The isoamyl group is the same as an isopentyl or 3-methylbutyl group: (d) N,N-dimethylbenzamide 21.3 The E and Z conformations of N-acetylproline: 21.5 As shown by the data above the problem, a carboxylic acid has a higher boiling point than an ester because it can both donate and accept hydrogen bonds within its liquid state; hydrogen bonding does not occur in the ester. Consequently, pentanoic acid (valeric acid) has a higher boiling point than methyl butanoate. Here are the actual data: INSTRUCTOR SUPPLEMENTAL SOLUTIONS TO PROBLEMS • CHAPTER 21 2 21.7 (a) The carbonyl absorption of the ester occurs at higher frequency, and only the carboxylic acid has the characteristic strong, broad O—H stretching absorption in 2400–3600 cm–1 region. (d) In N-methylpropanamide, the N-methyl group is a doublet at about d 3. N-Ethylacetamide has no doublet resonances. In N-methylpropanamide, the a-protons are a quartet near d 2.5. In N-ethylacetamide, the a- protons are a singlet at d 2. The NMR spectrum of N-methylpropanamide has no singlets. 21.9 (a) The first ester is more basic because its conjugate acid is stabilized not only by resonance interaction with the ester oxygen, but also by resonance interaction with the double bond; that is, the conjugate acid of the first ester has one more important resonance structure than the conjugate acid of the second. -

Metabolic-Hydroxy and Carboxy Functionalization of Alkyl Moieties in Drug Molecules: Prediction of Structure Influence and Pharmacologic Activity
molecules Review Metabolic-Hydroxy and Carboxy Functionalization of Alkyl Moieties in Drug Molecules: Prediction of Structure Influence and Pharmacologic Activity Babiker M. El-Haj 1,* and Samrein B.M. Ahmed 2 1 Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences, University of Science and Technology of Fujairah, Fufairah 00971, UAE 2 College of Medicine, Sharjah Institute for Medical Research, University of Sharjah, Sharjah 00971, UAE; [email protected] * Correspondence: [email protected] Received: 6 February 2020; Accepted: 7 April 2020; Published: 22 April 2020 Abstract: Alkyl moieties—open chain or cyclic, linear, or branched—are common in drug molecules. The hydrophobicity of alkyl moieties in drug molecules is modified by metabolic hydroxy functionalization via free-radical intermediates to give primary, secondary, or tertiary alcohols depending on the class of the substrate carbon. The hydroxymethyl groups resulting from the functionalization of methyl groups are mostly oxidized further to carboxyl groups to give carboxy metabolites. As observed from the surveyed cases in this review, hydroxy functionalization leads to loss, attenuation, or retention of pharmacologic activity with respect to the parent drug. On the other hand, carboxy functionalization leads to a loss of activity with the exception of only a few cases in which activity is retained. The exceptions are those groups in which the carboxy functionalization occurs at a position distant from a well-defined primary pharmacophore. Some hydroxy metabolites, which are equiactive with their parent drugs, have been developed into ester prodrugs while carboxy metabolites, which are equiactive to their parent drugs, have been developed into drugs as per se. -

CO2 Capture from Flue Gas Using Amino Acid Salt Solutions
CO2 capture from flue gas using amino acid salt solutions Jacco van Holst, Patricia. P. Politiek, John P. M. Niederer, Geert F. Versteeg* University of Twente, Faculty Science and Technology (UT TNW), P.O. Box 217, 7500 AE Enschede, The Netherlands Abstract An initial kinetic study was performed on the reaction of CO2 with various potassium amino acid salt solutions at 298 K. Kinetics were measured at 0.5 kmol/m3, reason for which only apparent ki- netic constants are presented. The results were compared with the work of Kumar et al. [1] and Penny and Ritter [2]. Keywords: CO2, flue gas treatment, kinetics, potassium amino acid salt solutions Introduction One of the most alarming global environmental problems of today is the increase of the natural greenhouse effect. This problem is mainly caused by the increasing atmospheric carbon dioxide concentration due to the burning of fossil fuels for power generation. To reduce these problems, the carbon dioxide emissions from flue and fuel gases produced in combustion and gasification proc- esses in power plants have to be decreased by efficiency improvements and carbon dioxide capture. The removal of acid gases such as carbon dioxide, H2S or COS by absorption in aqueous alkanola- mine solutions is widely used in the chemical industry. Carbon dioxide reacts with primary and sec- ondary amines, reaching an equilibrium of carbamate, bicarbonate, and carbonate species. The ini- tial absorption reaction is the formation of the carbamate, which can then undergo hydrolysis to the bicarbonate and, if conditions such as pH are suitable, the carbonate species. The degree of hydroly- sis of the carbamate is determined by parameters such as amine concentration, solution pH, and the chemical stability of the carbamate [3]. -

The Relative Rates of Thiol–Thioester Exchange and Hydrolysis for Alkyl and Aryl Thioalkanoates in Water
Orig Life Evol Biosph (2011) 41:399–412 DOI 10.1007/s11084-011-9243-4 PREBIOTIC CHEMISTRY The Relative Rates of Thiol–Thioester Exchange and Hydrolysis for Alkyl and Aryl Thioalkanoates in Water Paul J. Bracher & Phillip W. Snyder & Brooks R. Bohall & George M. Whitesides Received: 14 April 2011 /Accepted: 16 June 2011 / Published online: 5 July 2011 # Springer Science+Business Media B.V. 2011 Abstract This article reports rate constants for thiol–thioester exchange (kex), and for acid- mediated (ka), base-mediated (kb), and pH-independent (kw) hydrolysis of S-methyl thioacetate and S-phenyl 5-dimethylamino-5-oxo-thiopentanoate—model alkyl and aryl thioalkanoates, respectively—in water. Reactions such as thiol–thioester exchange or aminolysis could have generated molecular complexity on early Earth, but for thioesters to have played important roles in the origin of life, constructive reactions would have needed to compete effectively with hydrolysis under prebiotic conditions. Knowledge of the kinetics of competition between exchange and hydrolysis is also useful in the optimization of systems where exchange is used in applications such as self-assembly or reversible binding. For the alkyl thioester S-methyl thioacetate, which has been synthesized in −5 −1 −1 −1 −1 −1 simulated prebiotic hydrothermal vents, ka = 1.5×10 M s , kb = 1.6×10 M s , and −8 −1 kw = 3.6×10 s . At pH 7 and 23°C, the half-life for hydrolysis is 155 days. The second- order rate constant for thiol–thioester exchange between S-methyl thioacetate and 2- −1 −1 sulfonatoethanethiolate is kex = 1.7 M s . -

Chapter 7, Haloalkanes, Properties and Substitution Reactions of Haloalkanes Table of Contents 1
Chapter 7, Haloalkanes, Properties and Substitution Reactions of Haloalkanes Table of Contents 1. Alkyl Halides (Haloalkane) 2. Nucleophilic Substitution Reactions (SNX, X=1 or 2) 3. Nucleophiles (Acid-Base Chemistry, pka) 4. Leaving Groups (Acid-Base Chemistry, pka) 5. Kinetics of a Nucleophilic Substitution Reaction: An SN2 Reaction 6. A Mechanism for the SN2 Reaction 7. The Stereochemistry of SN2 Reactions 8. A Mechanism for the SN1 Reaction 9. Carbocations, SN1, E1 10. Stereochemistry of SN1 Reactions 11. Factors Affecting the Rates of SN1 and SN2 Reactions 12. --Eliminations, E1 and E2 13. E2 and E1 mechanisms and product predictions In this chapter we will consider: What groups can be replaced (i.e., substituted) or eliminated The various mechanisms by which such processes occur The conditions that can promote such reactions Alkyl Halides (Haloalkane) An alkyl halide has a halogen atom bonded to an sp3-hybridized (tetrahedral) carbon atom The carbon–chlorine and carbon– bromine bonds are polarized because the halogen is more electronegative than carbon The carbon-iodine bond do not have a per- manent dipole, the bond is easily polarizable Iodine is a good leaving group due to its polarizability, i.e. its ability to stabilize a charge due to its large atomic size Generally, a carbon-halogen bond is polar with a partial positive () charge on the carbon and partial negative () charge on the halogen C X X = Cl, Br, I Different Types of Organic Halides Alkyl halides (haloalkanes) sp3-hybridized Attached to Attached to Attached -
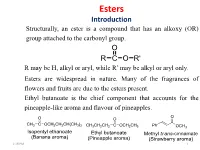
Esters Introduction Structurally, an Ester Is a Compound That Has an Alkoxy (OR) Group Attached to the Carbonyl Group
Esters Introduction Structurally, an ester is a compound that has an alkoxy (OR) group attached to the carbonyl group. O R C O R' R may be H, alkyl or aryl, while R’ may be alkyl or aryl only. Esters are widespread in nature. Many of the fragrances of flowers and fruits are due to the esters present. Ethyl butanoate is the chief component that accounts for the pineapple-like aroma and flavour of pineapples. 1:18 PM 1 Nomenclature of Esters Names of esters consist of two words that reflect the composite structure of the ester. The first word is derived from the alkyl group of the alcohol component, and the second word from the carboxylate group of the carboxylic acid component of the ester. The name of the carboxylate portion is derived by substituting the -ic acid suffix of the parent carboxylic acid with the –ate suffix. The alkyl group is cited first followed by the carboxylate group separated by a space. An ester is thus named as an alkyl 1:18 PM alkanoate. 2 IUPAC Nomenclature of Esters Examples 1:18 PM 3 Synthesis of Esters Preparative Strategies Highlighted below are some of the most common strategies by which esters are prepared. The esters are commonly prepared from the reaction of carboxylic acids, acid chlorides and acid anhydrides with alcohols. 1:18 PM 4 Synthesis of Esters Acid-Catalysed Esterification of a Carboxylic Acid and an Alcohol The acid-catalysed reaction of carboxylic acids and alcohols provides esters. Typically, a catalytic amount of a strong inorganic (mineral) acid such as H2SO4, HCl and H3PO4 is used. -

SALTS of FATTY ACIDS
SALTS of FATTY ACIDS Prepared at the 33rd JECFA (1988), published in FNP 38 (1988) and in FNP 52 (1992). Metals and arsenic specifications revised at the 55th JECFA (2000). An ADI 'not specified' was established at the 33rd JECFA (1988) SYNONYMS INS No. 470 DEFINITION These products consist of calcium, potassium or sodium salts of commercial myristic, oleic, palmitic, stearic, acids or mixtures of these acids from edible fats and oils. The article of commerce can be further specified by: - saponification value, - solidification point for the fatty acids obtained from the salts, - iodine value, - residue on ignition including assay of the cation, and - moisture content Assay Not less than 95% total fatty acid salts, dry weight basis DESCRIPTION Hard, white or faintly yellowish, somewhat glossy and crystalline solids or semi-solids or white or yellowish-white powder FUNCTIONAL USES Anticaking agent, emulsifier CHARACTERISTICS IDENTIFICATION Solubility (Vol. 4) Potassium and sodium salts are soluble in water and ethanol; calcium salts are insoluble in water, ethanol and ether Test for cations Heat 1 g of the sample with a mixture of 25 ml of water and 5 ml of hydrochloric acid. Fatty acids are liberated, floating as a solid or oil layer on the surface which is soluble in hexane. After cooling, aqueous layer is decanted and evaporated to dryness. Dissolve the residue in water and test for the appropriate cation. Fatty acid composition Using the Method of Assay, identify the individual fatty sample. The fatty acid(s) in primary abundance should conform to those declared on the label of the product PURITY Free fatty acids Not more than 3% Measure free fatty acids as directed in the method Free Fatty Acids. -

Fermentation and Ester Taints
Fermentation and Ester Taints Anita Oberholster Introduction: Aroma Compounds • Grape‐derived –provide varietal distinction • Yeast and fermentation‐derived – Esters – Higher alcohols – Carbonyls – Volatile acids – Volatile phenols – Sulfur compounds What is and Esters? • Volatile molecule • Characteristic fruity and floral aromas • Esters are formed when an alcohol and acid react with each other • Few esters formed in grapes • Esters in wine ‐ two origins: – Enzymatic esterification during fermentation – Chemical esterification during long‐term storage Ester Formation • Esters can by formed enzymatically by both the plant and microbes • Microbes – Yeast (Non‐Saccharomyces and Saccharomyces yeast) – Lactic acid bacteria – Acetic acid bacteria • But mainly produced by yeast (through lipid and acetyl‐CoA metabolism) Ester Formation Alcohol function Keto acid‐Coenzyme A Ester Ester Classes • Two main groups – Ethyl esters – Acetate esters • Ethyl esters = EtOH + acid • Acetate esters = acetate (derivative of acetic acid) + EtOH or complex alcohol from amino acid metabolism Ester Classes • Acetate esters – Ethyl acetate (solvent‐like aroma) – Isoamyl acetate (banana aroma) – Isobutyl acetate (fruit aroma) – Phenyl ethyl acetate (roses, honey) • Ethyl esters – Ethyl hexanoate (aniseed, apple‐like) – Ethyl octanoate (sour apple aroma) Acetate Ester Formation • 2 Main factors influence acetate ester formation – Concentration of two substrates acetyl‐CoA and fusel alcohol – Activity of enzyme responsible for formation and break down reactions • Enzyme activity influenced by fermentation variables – Yeast – Composition of fermentation medium – Fermentation conditions Acetate/Ethyl Ester Formation – Fermentation composition and conditions • Total sugar content and optimal N2 amount pos. influence • Amount of unsaturated fatty acids and O2 neg. influence • Ethyl ester formation – 1 Main factor • Conc. of precursors – Enzyme activity smaller role • Higher fermentation temp formation • C and N increase small effect Saerens et al. -

DP70460.Pdf (4.893Mb)
HEAT CHAHGES ACCGKPABYIIJG ABSGRFTIOH EQUILIBRIA IB SOLTJTIQH. D. C. UCETSBVAUfER C h e w . LD Sill ,/V\7 od L VVC\ I If) <2 D-C. C o ' :o Thesim submitted to the Faculty of the Graduate School of the TJnivsrsity of Maryland in partial fulfillment of the requirements for the degree of Doctor of Phil osophy* A 1 9 fc 6 .. UMI Number: DP70460 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. Dissertation Publishing UMI DP70460 Published by ProQuest LLC (2015). Copyright in the Dissertation held by the Author. Microform Edition © ProQuest LLC. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 ACKHQWLXDGMEHT The writer wishes to express hie appreciation and thaiifcs to Dr* Hell S. Gordon, Head of the Depart ment of Chemistry of the University of Maryland who dlreetcd this work and gate helpful suggestions and advice* TABLE OF G CH U M S X* Introduct 1 q h -—-*——— «*►»»««*«*»*«»»*— «■»»*-«««»»» x . Historical He view-----— ----- —■—— — — Z Heat of Absorption of Liquids art Gases- 1 4 Heat of Coagulation --- -- ™ -i^ * General Methods and Materials--— — — — — 2H of G els— 38H ----- - --- — ;S6 ------ Froeebnre——— ——-——32 If# Experiiaenial Hes’nlts-— --— — — — — ---- g*> T. Hubimavy C oixdua i OHS— —— — -— —— 61 FX* Lit eratare Gi ted :——— Introduction Adscript ion has for a long time teen a well recognised phenomenon and numerous investigations have been mad® on the nature and magnitude of the energy changes involved* Some of the measurementa on the adsorption of gases have shown ti&se changes to be enormous. -

Reduction of Organic Functional Groups Using Hypophosphites Rim Mouselmani
Reduction of Organic Functional Groups Using Hypophosphites Rim Mouselmani To cite this version: Rim Mouselmani. Reduction of Organic Functional Groups Using Hypophosphites. Other. Univer- sité de Lyon; École Doctorale des Sciences et de Technologie (Beyrouth), 2018. English. NNT : 2018LYSE1241. tel-02147583v2 HAL Id: tel-02147583 https://tel.archives-ouvertes.fr/tel-02147583v2 Submitted on 5 Jun 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THESE de DOCTORAT DE L’UNIVERSITE DE LYON EN COTUTELLE AVEC L'UNIVERSITÉ LIBANAISE opérée au sein de l’Université Claude Bernard Lyon 1 École Doctorale de Chimie-École Doctorale des Sciences et Technologies Discipline : Chimie Soutenue publiquement le 07/11/2018, par Rim MOUSELMANI Reduction of Organic Functional Groups Using Hypophosphites Devant le jury composé de Mme. Micheline DRAYE Université Savoie Mont Blanc Rapporteure M. Mohammad ELDAKDOUKI Université Arabe de Beyrouth Rapporteur Mme. Emmanuelle SCHULZ Université Paris 11 examinatrice M. Abderrahmane AMGOUNE Université Lyon 1 Président M. Mahmoud FARAJ Université Internationale Libanaise examinateur Mme. Estelle MÉTAY Université Lyon 1 Directrice de thèse M. Ali HACHEM Université Libanaise Directeur de thèse M. Marc LEMAIRE Université Lyon 1 Membre invité M. -

Isopropyl Acetate Iac
ISOPROPYL ACETATE IAC CAUTIONARY RESPONSE INFORMATION 4. FIRE HAZARDS 7. SHIPPING INFORMATION 4.1 Flash Point: 60°F O.C. 37°F C.C. 7.1 Grades of Purity: 95-99+% Common Synonyms Watery liquid Colorless Pleasant fruity 4.2 Flammable Limits in Air: 1.8%-8.0% 7.2 Storage Temperature: Ambient Acetic acid, isopropyl ester odor 4.3 Fire Extinguishing Agents: Alcohol foam, 2-Propyl acetate 7.3 Inert Atmosphere: No requirement dry chemical, carbon dioxide 7.4 Venting: Open (flame arrester) or pressure- Floats and mixes slowly with water. Flammable, irritating vapor is 4.4 Fire Extinguishing Agents Not to Be vacuum produced. Used: Not pertinent 7.5 IMO Pollution Category: Currently not available 4.5 Special Hazards of Combustion Keep people away. Products: Not pertinent 7.6 Ship Type: Currently not available Shut off ignition sources and call fire department. 4.6 Behavior in Fire: Not pertinent 7.7 Barge Hull Type: Currently not available Stay upwind and use water spray to ``knock down'' vapor. Avoid contact with liquid and vapor. 4.7 Auto Ignition Temperature: 860°F Notify local health and pollution control agencies. 4.8 Electrical Hazards: Not pertinent 8. HAZARD CLASSIFICATIONS Protect water intakes. 4.9 Burning Rate: Currently not available 8.1 49 CFR Category: Flammable liquid 4.10 Adiabatic Flame Temperature: Currently 8.2 49 CFR Class: 3 FLAMMABLE. not available Fire Flashback along vapor trail may occur. 8.3 49 CFR Package Group: Not listed. 4.11 Stoichometric Air to Fuel Ratio: 30.9 Vapor may explode if ignited in an enclosed area. -

APPENDIX G Acid Dissociation Constants
harxxxxx_App-G.qxd 3/8/10 1:34 PM Page AP11 APPENDIX G Acid Dissociation Constants § ϭ 0.1 M 0 ؍ (Ionic strength ( † ‡ † Name Structure* pKa Ka pKa ϫ Ϫ5 Acetic acid CH3CO2H 4.756 1.75 10 4.56 (ethanoic acid) N ϩ H3 ϫ Ϫ3 Alanine CHCH3 2.344 (CO2H) 4.53 10 2.33 ϫ Ϫ10 9.868 (NH3) 1.36 10 9.71 CO2H ϩ Ϫ5 Aminobenzene NH3 4.601 2.51 ϫ 10 4.64 (aniline) ϪO SNϩ Ϫ4 4-Aminobenzenesulfonic acid 3 H3 3.232 5.86 ϫ 10 3.01 (sulfanilic acid) ϩ NH3 ϫ Ϫ3 2-Aminobenzoic acid 2.08 (CO2H) 8.3 10 2.01 ϫ Ϫ5 (anthranilic acid) 4.96 (NH3) 1.10 10 4.78 CO2H ϩ 2-Aminoethanethiol HSCH2CH2NH3 —— 8.21 (SH) (2-mercaptoethylamine) —— 10.73 (NH3) ϩ ϫ Ϫ10 2-Aminoethanol HOCH2CH2NH3 9.498 3.18 10 9.52 (ethanolamine) O H ϫ Ϫ5 4.70 (NH3) (20°) 2.0 10 4.74 2-Aminophenol Ϫ 9.97 (OH) (20°) 1.05 ϫ 10 10 9.87 ϩ NH3 ϩ ϫ Ϫ10 Ammonia NH4 9.245 5.69 10 9.26 N ϩ H3 N ϩ H2 ϫ Ϫ2 1.823 (CO2H) 1.50 10 2.03 CHCH CH CH NHC ϫ Ϫ9 Arginine 2 2 2 8.991 (NH3) 1.02 10 9.00 NH —— (NH2) —— (12.1) CO2H 2 O Ϫ 2.24 5.8 ϫ 10 3 2.15 Ϫ Arsenic acid HO As OH 6.96 1.10 ϫ 10 7 6.65 Ϫ (hydrogen arsenate) (11.50) 3.2 ϫ 10 12 (11.18) OH ϫ Ϫ10 Arsenious acid As(OH)3 9.29 5.1 10 9.14 (hydrogen arsenite) N ϩ O H3 Asparagine CHCH2CNH2 —— —— 2.16 (CO2H) —— —— 8.73 (NH3) CO2H *Each acid is written in its protonated form.