Isopropyl Acetate Iac
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Isoamyl Acetate
SUMMARY OF DATA FOR CHEMICAL SELECTION Isoamyl Acetate CAS No. 123-92-2 Prepared for NTP by Technical Resources International, Inc Prepared on 11/94 Under NCI Contract No. N01-CP-56019 Table of Contents I. Chemical Identification II. Exposure Information Table 1. Levels of isoamyl acetate reported in foods III. Evidence for Possible Carcinogenic Activity Appendix A: Structural Analogs of Isoamyl Acetate IV. References SUMMARY OF DATA FOR CHEMICAL SELECTION CHEMICAL IDENTIFICATION CAS Registry No.: 123-92-2 Chem. Abstr. Name: 1-Butanol, 3-methyl-, acetate Synonyms: Acetic acid 3-methylbutyl ester; acetic acid, isopentyl ester; AI3-00576; banana oil; isoamyl ethanoate; isopentyl acetate; isopentyl alcohol, acetate; pear oil; 3-methyl-1-butanol acetate; 3-methyl-1-butyl acetate; 3-methylbutyl acetate; 3-methylbutyl ethanoate; i-amyl acetate Structure: Molecular Formula and Molecular Weight: C7H14O2 Mol. Wt.: 130.18 Chemical and Physical Properties: Description: Colorless, flammable liquid with a banana-like odor (ACGIH, 1993). Boiling Point: 142°C (Lide, 1993) Melting Point: -78.5°C (Mark, et al, 1984; Lide, 1993) Solubility: Soluble in water (2000 mg/L at 25°C) (Howard, 1990); soluble in ethanol, diethyl ether, and acetone (Lide, 1993). Vapor 4.5 mm Hg at 20°C (Howard, 1990) Pressure: Refractive 1.4003 (Lide, 1993) Index: Flash Point: closed cup, 33°C; open cup, 38°:C (Budavari, 1989) Density: 0.876 (Lewis, 1993) Reactivity: Thermal decomposition of isoamyl acetate may produce acrid fumes. Contact with strong oxidizing agents, strong acids, and alkaline materials should be avoided (Haarmann & Reimer Corp., 1994). Hazardous decomposition products of isoamyl acetate include CO and CO2 (AESAR/Alfa, 1994) Log 2.13 (Howard, 1990) P(octanol/water partition coefficient): Technical Isoamyl acetate is commercially available as both a natural and synthetic product with a purity Products and range of 95-99+%. -

Comprehensive Characterization of Toxicity of Fermentative Metabolites on Microbial Growth Brandon Wilbanks1 and Cong T
Wilbanks and Trinh Biotechnol Biofuels (2017) 10:262 DOI 10.1186/s13068-017-0952-4 Biotechnology for Biofuels RESEARCH Open Access Comprehensive characterization of toxicity of fermentative metabolites on microbial growth Brandon Wilbanks1 and Cong T. Trinh1,2* Abstract Background: Volatile carboxylic acids, alcohols, and esters are natural fermentative products, typically derived from anaerobic digestion. These metabolites have important functional roles to regulate cellular metabolisms and broad use as food supplements, favors and fragrances, solvents, and fuels. Comprehensive characterization of toxic efects of these metabolites on microbial growth under similar conditions is very limited. Results: We characterized a comprehensive list of thirty-two short-chain carboxylic acids, alcohols, and esters on microbial growth of Escherichia coli MG1655 under anaerobic conditions. We analyzed toxic efects of these metabo- lites on E. coli health, quantifed by growth rate and cell mass, as a function of metabolite types, concentrations, and physiochemical properties including carbon number, chemical functional group, chain branching feature, energy density, total surface area, and hydrophobicity. Strain characterization revealed that these metabolites exert distinct toxic efects on E. coli health. We found that higher concentrations and/or carbon numbers of metabolites cause more severe growth inhibition. For the same carbon numbers and metabolite concentrations, we discovered that branched chain metabolites are less toxic than the linear chain ones. Remarkably, shorter alkyl esters (e.g., ethyl butyrate) appear less toxic than longer alkyl esters (e.g., butyl acetate). Regardless of metabolites, hydrophobicity of a metabolite, gov- erned by its physiochemical properties, strongly correlates with the metabolite’s toxic efect on E. coli health. -

BLUE BOOK 1 Methyl Acetate CIR EXPERT PANEL MEETING
BLUE BOOK 1 Methyl Acetate CIR EXPERT PANEL MEETING AUGUST 30-31, 2010 Memorandum To: CIR Expert Panel Members and Liaisons From: Bart Heldreth Ph.D., Chemist Date: July 30, 2010 Subject: Draft Final Report of Methyl Acetate, Simple Alkyl Acetate Esters, Acetic Acid and its Salts as used in Cosmetics . This review includes Methyl Acetate and the following acetate esters, relevant metabolites and acetate salts: Propyl Acetate, Isopropyl Acetate, t-Butyl Acetate, Isobutyl Acetate, Butoxyethyl Acetate, Nonyl Acetate, Myristyl Acetate, Cetyl Acetate, Stearyl Acetate, Isostearyl Acetate, Acetic Acid, Sodium Acetate, Potassium Acetate, Magnesium Acetate, Calcium Acetate, Zinc Acetate, Propyl Alcohol, and Isopropyl Alcohol. At the June 2010 meeting, the Panel reviewed information submitted in response to an insufficient data announcement for HRIPT data for Cetyl Acetate at the highest concentration of use (lipstick). On reviewing the data in the report, evaluating the newly available unpublished studies and assessing the newly added ingredients, the Panel determined that the data are now sufficient, and issued a Tentative Report, with a safe as used conclusion. Included in this report are Research Institute for Fragrance Materials (RIFM) sponsored toxicity studies on Methyl Acetate and Propyl Acetate, which were provided in “wave 2” at the June Panel Meeting but are now incorporated in full. The Tentative Report was issued for a 60 day comment period (60 days as of the August panel meeting start date). The Panel should now review the Draft Final Report, confirm the conclusion of safe, and issue a Final Report. All of the materials are in the Panel book as well as in the URL for this meeting's web page http://www.cir- safety.org/aug10.shtml. -

Syringe Filters Solvent Compatibility Chart
Syringe Filters Solvent Compatibility Chart Group of Substance & Cellulose Nylon PES PTFE PVDF Chemical Reagents Acetate ACIDS Acetic, 5% L R R R R Acetic, 10% L R R R R Acetic, 25% N L R R R Acetic, Glacial N N R R R Boric - L - R - Formic 25% L N - R - Hydrochloric 15% L L R R L Hydrochloric 25% N N R R - Hydrochloric concentrated N N L R N Hydrofluoric 10% N N - - - Hydrofluoric 35% N N - R - Nitric 25% N N R R - Nitric 6N, 38% N N L R R Top 10 Reasons to Use Restek Syringe Filters Nitric concentrated N N N R N Phosphoric 25% L N R R - 1 Protect any analytical system. Sulfuric 25% N N N R - 2 Extend LC column lifetime. Sulfuric 6N, 29% N N N R - 3 Achieve more reproducible analyses. Sulfuric concentrated N N N R N Trichloroacetic 10% N N - R R 4 Variety of membranes, porosities, and diameters available. ALKALINES 5 Luer lock inlet provides strong, leak-tight syringe connection to Ammonium Hydroxide 25% N R R R L withstand filtration pressure. Formalin 30% L L R - - 6 Rugged polypropylene construction— autoclavable to 121 °C for Sodium Hydroxide 3N, 12% N R R R R 15 minutes (75 psi). ALCOHOLS 7 Color coded by membrane for easy identification. Amyl Alcohol L R N R R 8 Convenient dispenser box. Benzyl Alcohol L L L L L 9 FREE sample pack available. Contact your Restek sales Butyl Alcohol L R L R R representative today. -

DESIGN of a FLEXIBLE PROCESS for the PRODUCTION of N-BUTYL ACETATE and ISOPROPYL ACETATE
Department of Chemical Engineering, Auburn University CHEN 4470, Spring 2012 DESIGN OF A FLEXIBLE PROCESS FOR THE PRODUCTION OF n-BUTYL ACETATE AND ISOPROPYL ACETATE 1. Background Esters are widespread in nature and are responsible for the aroma of many fruits including apples, pears, bananas, pineapples and strawberries. This characteristic has led to extensive use of esters in the fragrance and flavor industries. Furthermore, ester bonds are also found in many polymers and several million tons of polyesters are produced industrially every year. This project involves the production of two different esters: A) n-Butyl Acetate (BuAc) - a chemical used as a solvent for various chemicals including inks, paints, and lacquers; and B) iso-Propyl Acetate (IPAc) – a solvent for cellulose, plastics, oil and fats, and a ingredient in some printing inks and perfumes. 2. Design Objectives In this project, you will utilize your process design knowledge to develop candidate process flowsheets from the initial concept through material balances, equipment sizing, control system design, and economic analysis. The processes should include all equipment needed to produce the products. A given process may include (but is not limited to) reactors, columns, heat exchangers, extractors, decanters, reflux pots, pumps, storage tanks. The primary objective of this project is to design a grass-roots facility capable of producing a total of 10,000 metric tons per year of 99.5 wt% Butyl Acetate. Due to similarities in the reaction schemes and to provide the ability to adapt to changes in market demand, a secondary objective is to evaluate the retrofitting requirements (if any) for switching the Butyl Acetate facility to produce 99.5 wt% iso-Propyl Acetate instead. -

Isopropyl Acetate Secondary Propyl Acetate Acetic Acid, Isopropyl Ester Isopropyl Ethanoate
Product Information Isopropyl Acetate Secondary Propyl Acetate Acetic Acid, Isopropyl Ester Isopropyl Ethanoate (CH3)2CHOC(O)CH3 Description Physical properties A colorless liquid with an aromatic Molecular Weight 102.13 fruity odor with moderate solubility in water and a low flash point. Relative Evaporation Rate nBuAc=1 5 ° Vapor Pressure at 20 C, mmHg 47.0 ° Density at 20 C lb/gal 7.28 ° Specific Gravity at 20/20 C 0.869 ° Viscosity at 20 C cP 0.5 Surface Tension (dynes/cm at 20°C) 22.3 ° (dynes/cm at 25 C) - Hansen Solubility Parameters, [cal/cm3]1/2 Total 8.6 Non-Polar 7.3 Polar 2.2 Hydrogen Bonding 4.0 ° Boiling Point, C at 760mm Hg 88.5 Solubility at 20°C %Wt In Water 3 %Wt Water in 1.9 ° Closed Cup Flash Point F36 † SARA 313 (see note 1 )N †† Hazardous Air Pollutant (see note 2 )N † Note 1: Superfund Amendments and Reauthorization Act of 1986 (SARA) Title III Section 313 †† Note 2: Hazardous Air Pollutants listed under Title III of the Clean Air Act Classification/Registry Numbers CAS Number 108-21-4 (Please see second page) EINECS 203-5611 DOW RESTRICTED - For internal use only*Trademark of The Dow Chemical Company Isopropyl Acetate Secondary Propyl Acetate Acetic Acid Isopropyl Ester Features • Non-HAP (Hazardous air pollutant) Solvent • Good resin solvent • Mild odor • Fast evaporating Applications • Coatings • Cleaning fluids • Printing inks • Cosmetic / personal care solvent • Fragrance solvent How supplied Region Packaging Transport Mode Europe/Africa Bulk or Drum Isotank/Marine Vessel/Tank Truck/Package Truck Latin America -
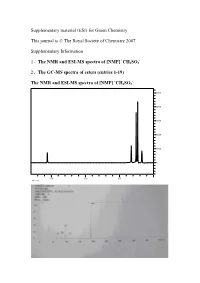
And the GC-MS
Supplementary material (ESI) for Green Chemistry This journal is © The Royal Society of Chemistry 2007 Supplementary Information + - 1、The NMR and ESI-MS spectra of [NMP] CH3SO3 2、The GC-MS spectra of esters (entries 1-19) + - The NMR and ESI-MS spectra of [NMP] CH3SO3 5000 4000 3000 2000 1000 0 15.0 10.0 5.0 ppm (t1) The GC-MS spectra of esters (entries 1-19) (1) The GC-MS spectrum of propyl acetate 0.69min, solvent of chloroform; 0.97min, propyl acetate The MS spectra of propyl acetate (0.97min) (2) The GC-MS spectrum of isopropyl acetate 1.98min, isopropyl acetate The MS spectrum of isopropyl acetate (1.98min) (3) The GC-MS spectrum of butyl acetate 2.90min, butyl acetate 4(ZHANG) #665 RT: 3.61 AV: 1 NL: 2.56E7 T: + c Full ms [ 14.00-400.00] 72.85 100 90 80 70 85.85 41.88 60 50 40 Relative Abundance 57.88 30 20 100.93 10 135.97154.12 207.58 253.45 280.90 355.76 0 50 100 150 200 250 300 350 400 m/z The MS spectrum of butyl acetate (2.90min) (4) The GC-MS spectrum of isopentyl acetate 2.20min, isopentyl acetate 3(ZHANG) #405 RT: 2.98 AV: 1 NL: 2.71E7 T: + c Full ms [ 14.00-400.00] 86.89 100 90 80 70 60 41.86 50 40 85.90 Relative Abundance Relative 57.85 30 20 69.89 10 87.91 88.94 121.16 158.00 207.11 226.90 280.98 314.33 356.16 0 50 100 150 200 250 300 350 400 m/z The MS spectrum of isopentyl acetate (2.2min) (5) The GC-MS spectrum of pentyl acetate 3.54min, pentyl acetate The MS spectrum of pentyl acetate (3.54min) (6) The GC-MS spectrum of hexyl acetate 4.15min, hexyl acetate 1 #920 RT: 3.08 AV: 1 NL: 5.24E7 T: + c Full ms [ 45.00-400.00] -

Isopropyl Acetate Safety Data Sheet According to Regulation (EU) 2015/830 Date of Issue: 7/27/2018 Revision Date: 7/27/2018 Supersedes: 6/30/2015
isopropyl acetate Safety Data Sheet according to Regulation (EU) 2015/830 Date of issue: 7/27/2018 Revision date: 7/27/2018 Supersedes: 6/30/2015 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product form : Substance Chemical name : isopropyl acetate EC Index-No. : 607-024-00-6 EC-No. : 203-561-1 CAS-No. : 108-21-4 REACH registration No : 01-2119537214-46-0003 Product code : ER09750010 Type of product : Pure substance Formula : C5H10O2 Synonyms : 1-methylethyl acetate / 2-acetoxypropane / 2-methyl ethyl ethanoate / 2-propyl acetate / 2- propyl ethanoate / acetate of isopropyl / acetic acid 1-methylethyl ester / acetic acid isopropyl ester / acetic acid, 1-methylethyl ester / IPAC / isopropyl acetate / secondary- propyl acetate / sec-propyl acetate Product group : Trade product BIG no : 50397 1.2. Relevant identified uses of the substance or mixture and uses advised against 1.2.1. Relevant identified uses Industrial/Professional use spec : Industrial For professional use only Use of the substance/mixture : Solvent Laboratory chemical Odorant 1.2.2. Uses advised against No additional information available 1.3. Details of the supplier of the safety data sheet Monument Chemical B.V.B.A. Ketenislaan 3 B-9130 Kallo - Belgium T +32 3 570 28 11 [email protected] - www.monumentchemical.com 1.4. Emergency telephone number Emergency number : BIG 24h/24h: +32 14 58 45 45 SECTION 2: Hazards identification 2.1. Classification of the substance or mixture Classification according to Regulation (EC) No. 1272/2008 [CLP] Flammable liquids, Category 2 H225 Serious eye damage/eye irritation, Category 2 H319 Specific target organ toxicity — Single exposure, Category 3, Narcosis H336 Full text of H statements : see section 16 Adverse physicochemical, human health and environmental effects Highly flammable liquid and vapour. -

Mixing Properties of Isopropyl Acetate+Aromatic Hydrocarbons at 298.15 K: Density, Refractive Index and Isentropic Compressibility
Korean J. Chem. Eng., 21(5), 1015-1025 (2004) Mixing Properties of Isopropyl Acetate+Aromatic Hydrocarbons at 298.15 K: Density, Refractive Index and Isentropic Compressibility J. M. Resa†, C. Gonzalez, E. Diez, R. G. Concha and M. Iglesias* Dpto. de Ingenieria Química, Universidad del Pais Vasco, Apto. 450, Vitoria, España *Departament d’Enginyeria Química, Escola Tècnica Superior d’Enginyeria Química, Universitat Rovira i Virgili, Avinguda Països Catalans 26, Campus Sescelades, 43007 Tarragona, España (Received 12 December 2003 • accepted 24 June 2004) Abstract−We present new experimental data of density, refractive index and speed of sound for the binaries of isopropyl acetate+(toluene, ethylbenzene, p-xylene, mesitylene, isopropylbenzene, butylbenzene, isobutylbenzene, or t-butylbenzene) at T=298.15 K and standard conditions, and the corresponding computed derived magnitudes (change of isentropic compressibility, change of refractive index on mixing, and excess molar volume). The mixtures show a clear expansive tendency for the highest molar weight compounds and the steric hindrance role of the aromatic chem- icals being analyzed to the light of the non-ideality on mixing. A good agreement among experimental data and the values estimated by theoretical procedures was obtained. Key words: Excess Volumes, Change of Refractive Indices, Change of Isentropic Compressibility, Isopropyl Acetate, Aromatic Hydrocarbon, Prediction INTRODUCTION compounds and the steric hindrance role of the aromatic chemicals being analyzed to explain the non-ideality of mixtures. In what is Many chemical, food and other industries present non-newto- referred to the as the estimation of these magnitudes, different pro- nian and non-ideal fluids and mixtures in their processes. -

Alcohols, Esters, and Oxo Acids Selection Chart
Alcohols, esters, and oxo acids selection chart Boiling Vapor Surface 3 1/2 Solubility Molecular Flash Evaporation Specific Density Viscosity Hansen solubility parameters (joules/cm ) point pressure, tension, wt% at 20°C Product Structural formula weight, point rate gravity at lb/gal cP at CAS number °C at mm dynes/cm g/mol °F / °C nBuAc=1 20/20°C at 20°C 20°C 760mm Hg Hg at 20°C at 20°C Dispersion Polar H bonding Total In water Water in a a a a Primary Amyl Alcohol Mixed Isomers C5H11OH (mixed isomers) 88.15 133.2 113/45 0.30 0.815 6.79 4.3 2.2 137-32-6 / 71-41-0 23.7 15.9 4.5 13.9 21.7 1.7 9.2 n-Butanol C4H9OH 74.12 117.7 97/36 0.44 0.810 6.75 3.0 4.9 71-36-3 24.8 16.0 5.7 15.8 23.1 7.7 20.1 2-Ethylhexanol CH3(CH2)3CH(C2H5)CH2OH 130.23 184.6 162/72 0.02 0.833 6.94 10.3 0.09 104-76-7 26.9 15.9 3.3 11.8 20.2 0.07 2.6 Isobutanol (CH3)2CHCH2OH 74.12 107.9 82/28 0.63 0.802 6.68 4.0 8.1 78-83-1 23.0 15.1 5.7 15.9 22.7 8.5 15 Alcohols Isopropanol (CH3)2CHOH 60.10 82.3 53/12 1.11 0.787 6.55 2.4 31.0 67-63-0 21.4 15.8 6.1 16.4 23.5 ∞ ∞ 2-Methyl Butanol C2H5CH(CH3)CH2OH 88.15 128.7 113/45 0.50 0.818 6.82 5.0 3.8 137-32-6 25.7 16.0 5.1 14.3 21.1 2.2 8.3 n-Pentanol C5H11OH 88.15 137.9 119/48 0.30 0.816 6.79 4.0 1.4 71-41-0 25.7 15.9 4.5 13.9 21.7 2 9.5 n-Propanol C3H7OH 60.10 97.2 73/23 0.87 0.816 6.71 2.2 15.2 71-23-8 23.8 16.0 6.8 17.4 24.5 ∞ ∞ b b b b b b Primary Amyl Acetate Mixed Isomers C5H11OC(O)CH3 (mixed isomers) 130.19 146.0 77/25 0.39 0.876 7.29 0.9 4 628-63-7 / 624-41-9 25.8 15.8 3.3 6.1 17.1 0.2 0.9 n-Butyl Acetate C4 H9OC(O)CH3 116.16 -

Names & Synonyms Pdf Icon[PDF – 126
V.c. INDEX OF NAMES AND SYNONYMS Example: The references to method title and number are listed in the following manner: ACETIC ACID, 1603 (Method Title) Ethanoic acid, see ACETIC ACID, 1603 (Synonym) Glacial acetic acid, see ACETIC ACID, 1603 (Synonym) Methane carboxylic acid, see ACETIC ACID, 1603 (Synonym) CAS #64-19-7, see ACETIC ACID, 1603 (Chemical Abstracts Registry Number) Acenaphthene, see POLYNUCLEAR AROMATIC HYDROCARBONS, 5506 (HPLC); 5515 (GC) Acenaphthylene, see POLYNUCLEAR AROMATIC HYDROCARBONS, 5506 (HPLC); 5515 (GC) ACETALDEHYDE, 2538 (GC), 3507 (HPLC), see ALDEHYDES, SCREENING, 2539; ALIPHATIC ALDEHYDES, 2018 ACETIC ACID, 1603 Acetic acid anhydride, see ACETIC ANHYDRIDE, 3506 Acetic acid ethyl ester, see ETHYL ACETATE, 1457 Acetic acid butyl ester, see ESTERS I, 1450 Acetic acid 1,1-dimethylethyl ester, see ESTERS I, 1450 Acetic acid ethylene glycol monoethyl ether ester, see ESTERS I, 1450 Acetic acid isobutyl ester, see ESTERS I, 1450 Acetic acid 3-methyl-1-butanol ester, see ESTERS I, 1450 Acetic acid methyl ester, see METHYL ACETATE, 1458 Acetic acid 4-methyl-2-pentanol ester, see ESTERS I, 1450 Acetic acid 1-methyl propyl ester, see ESTERS I, 1450 Acetic acid 1-pentanol ester, see ESTERS I, 1450 Acetic acid 2-pentanol ester, see ESTERS I, 1450 Acetic acid n-propyl ester, see ESTERS I, 1450 Acetic acid vinyl ester, see VINYL ACETATE, 1453 Acetic aldehyde, see ACETALDEHYDE (GC), 2538; 3507 (HPLC) ACETIC ANHYDRIDE, 3506 Acetic ether, see VOLATILE ORGANIC COMPOUNDS (SCREENING), 2549 ACETOIN, 2558 Acetone, see KETONES I, -

Identification of Organic Volatile Markers Associated with Aroma
molecules Article Identification of Organic Volatile Markers Associated with Aroma during Maturation of Strawberry Fruits Samuel Macario Padilla-Jiménez 1, María Valentina Angoa-Pérez 1, Hortencia Gabriela Mena-Violante 1, Guadalupe Oyoque-Salcedo 1, José Luis Montañez-Soto 1 and Ernesto Oregel-Zamudio 1,2,* 1 Instituto Politécnico Nacional, Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional (CIIDIR), Unidad Michoacán, Justo Sierra 28, Col. Centro, Jiquilpan 59510, Mexico; [email protected] (S.M.P.-J.); [email protected] (M.V.A.-P.); [email protected] (H.G.M.-V.); [email protected] (G.O.-S.); [email protected] (J.L.M.-S.) 2 Instituto Politécnico Nacional, Programa de Doctorado en Ciencias en Bioprocesos, Unidad Profesional Interdisciplinaria de Biotecnología (UPIBI), Av. Acueducto, Barrio la Laguna Ticoman, Ciudad de México 07340, Mexico * Correspondence: [email protected]; Tel.: +52-353-533-0218 Abstract: In the present study, organic volatile markers of three strawberry varieties (Albion, Fes- tival and Frontera) during the maturation process were investigated. Forty metabolites associated with aroma in fresh strawberries were monitored during seven stages of maturation using gas chromatography–mass spectrometry (GC-MS) equipped with headspace-solid phase microextraction (HS-SPME). The data were evaluated using multivariate analysis to observe correlations between the organic volatile compound profile and the seven phenological stages of maturation for each Citation: Padilla-Jiménez, S.M.; strawberry variety. The dynamic levels of butanoic acid methyl ester, hexanoic acid methyl ester, Angoa-Pérez, M.V.; Mena-Violante, octylcyclohexane, cyclohexane,1,1,2-trimethyl, linalool, tetradecane, and α-muurolene underwent H.G.; Oyoque-Salcedo, G.; distinctive changes in concentration during the maturation process.