ARCI Uniform Classification Guidelines for Foreign Substances, Or Similar State Regulatory Guidelines, Shall Be Assigned Points As Follows
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The 2021 List of Pharmacological Classes of Doping Agents and Doping Methods
BGBl. III - Ausgegeben am 8. Jänner 2021 - Nr. 1 1 von 23 The 2021 list of pharmacological classes of doping agents and doping methods www.ris.bka.gv.at BGBl. III - Ausgegeben am 8. Jänner 2021 - Nr. 1 2 von 23 www.ris.bka.gv.at BGBl. III - Ausgegeben am 8. Jänner 2021 - Nr. 1 3 von 23 THE 2021 PROHIBITED LIST WORLD ANTI-DOPING CODE DATE OF ENTRY INTO FORCE 1 January 2021 Introduction The Prohibited List is a mandatory International Standard as part of the World Anti-Doping Program. The List is updated annually following an extensive consultation process facilitated by WADA. The effective date of the List is 1 January 2021. The official text of the Prohibited List shall be maintained by WADA and shall be published in English and French. In the event of any conflict between the English and French versions, the English version shall prevail. Below are some terms used in this List of Prohibited Substances and Prohibited Methods. Prohibited In-Competition Subject to a different period having been approved by WADA for a given sport, the In- Competition period shall in principle be the period commencing just before midnight (at 11:59 p.m.) on the day before a Competition in which the Athlete is scheduled to participate until the end of the Competition and the Sample collection process. Prohibited at all times This means that the substance or method is prohibited In- and Out-of-Competition as defined in the Code. Specified and non-Specified As per Article 4.2.2 of the World Anti-Doping Code, “for purposes of the application of Article 10, all Prohibited Substances shall be Specified Substances except as identified on the Prohibited List. -
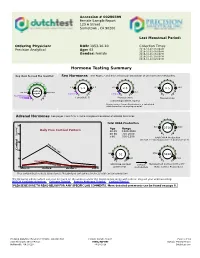
Hormone Testing Summary
Accession # 00280399 Female Sample Report 123 A Street Sometown , CA 90266 Last Menstrual Period: Ordering Physician: DOB: 1953-10-10 Collection Times: Precision Analytical Age: 63 2016-10-02 06:00AM 2016-10-02 08:00AM Gender: Female 2016-10-01 06:00PM 2016-10-01 10:00PM 2016-10-02 02:00AM Hormone Testing Summary Key (how to read the results): Sex Hormones See Pages 2 and 3 for a thorough breakdown of sex hormone metabolites opausa n l R e a m n e g 1.8 4.5 6.0 2.3 14.0 r 5.1 2.8 1.5 P e patient low limit high limit 20.0 result 0.2-0.7 0.3-2.0 Postmenopausal range Estradiol(E2) Progesterone Testosterone (Serum Equivalent, ng/mL) Progesterone Serum Equivalent is a calculated value based on urine pregnanediol. Adrenal Hormones See pages 4 and 5 for a more complete breakdown of adrenal hormones Total DHEA Production 300 500 3000 Age Range 2516 Daily Free Cortisol Pattern 20-39 1300-3000 240 40-60 750-2000 (ng/mg) >60 500-1200 Total DHEA Production (DHEAS + Etiocholanolone + Androsterone) 180 High Range Limit 120Cortisol 80 52 2750 5930 60 230 6500 Patient Values Low Range Limit 24hr Free Cortisol cortisol Metabolized Cortisol (THF+THE) 0 (A+B+C+D) metabolism (Total Cortisol Production) Waking (A) Morning (B) Afternoon (C) Night (D) Free cortisol best reflects tissue levels. Metabolized cortisol best reflects total cortisol production. The following videos (which can also be found on the website under the listed names along with others) may aid your understanding: DUTCH Complete Overview Estrogen Tutorial Female Androgen Tutorial Cortisol Tutorial PLEASE BE SURE TO READ BELOW FOR A NY SPECIFIC LA B COMMENTS. -

New Rules Pertaining to the Banning of Anabolic Steroids in the Western Australian Harness Racing Industry to Be Introduced 1St September 2014
NEW RULES PERTAINING TO THE BANNING OF ANABOLIC STEROIDS IN THE WESTERN AUSTRALIAN HARNESS RACING INDUSTRY TO BE INTRODUCED 1ST SEPTEMBER 2014 Notice is hereby given that the Board of Racing and Wagering WA have resolved that the RWWA Rules of Harness Racing 2004 be amended. In accordance with section 45 (1) (b) of the Racing and Wagering Western Australia Act 2003 the Board of Racing and Wagering WA on the 10th April 2014 resolved that these amendments be adopted accordingly into the RWWA Rules of Harness Racing. The Harness Racing Board had advised of these amendments and the RWWA Board has determined that these amendments will come into effect on 1st September 2014. The details of the relevant rules pertaining to this ban of anabolic steroids for reference can be found following this advice. There are many implications arising from the introduction of these rules, and to assist trainers and veterinarians to comply with the new rules the following explanatory statement has been prepared. Which steroids are banned under these rules? The new rules ban the use of "anabolic androgenic steroids" in Standardbred horses at any time from birth until retirement. "Anabolic androgenic steroids" include those that are currently registered in Australia by the APVMA for use in horses, such as boldenone, ethylestrenol (in Nitrotain), methandriol, nandrolone, stanozolol and testosterone. Exogenous anabolic androgenic steroids that are banned also include but are not limited to those listed in the WADA prohibited list, such as 1-androstenediol; 1-androstenedione; -

Classification of Medicinal Drugs and Driving: Co-Ordination and Synthesis Report
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Due date of deliverable: 21.07.2011 Actual submission date: 21.07.2011 Revision date: 21.07.2011 Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UVA Revision 0.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public PP Restricted to other programme participants (including the Commission x Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) DRUID 6th Framework Programme Deliverable D.4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Page 1 of 243 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Authors Trinidad Gómez-Talegón, Inmaculada Fierro, M. Carmen Del Río, F. Javier Álvarez (UVa, University of Valladolid, Spain) Partners - Silvia Ravera, Susana Monteiro, Han de Gier (RUGPha, University of Groningen, the Netherlands) - Gertrude Van der Linden, Sara-Ann Legrand, Kristof Pil, Alain Verstraete (UGent, Ghent University, Belgium) - Michel Mallaret, Charles Mercier-Guyon, Isabelle Mercier-Guyon (UGren, University of Grenoble, Centre Regional de Pharmacovigilance, France) - Katerina Touliou (CERT-HIT, Centre for Research and Technology Hellas, Greece) - Michael Hei βing (BASt, Bundesanstalt für Straßenwesen, Germany). -

The Steroid Metabolome in Men with Mood and Anxiety Disorders
Physiol. Res. 64 (Suppl. 2): S275-S282, 2015 https://doi.org/10.33549/physiolres.933067 The Steroid Metabolome in Men With Mood and Anxiety Disorders M. DUŠKOVÁ1, M. HILL1, M. BIČÍKOVÁ1, M. ŠRÁMKOVÁ1, D. ŘÍPOVÁ2, P. MOHR2, L. STÁRKA1 1Institute of Endocrinology, Prague, Czech Republic, 2National Institute of Mental Health, Klecany, Czech Republic Received May 5, 2015 Accepted May 20, 2015 Summary many other hormones and various factors have been The mood and behavior of individuals result from an orchestra of identified as modulators of mood and behavior in such many factors. Among them steroids play an important role; disorders. Recent studies have described the role of brain- however, only several common hormones have been investigated derived neurotrophic factor (BDNF) (Pluchino et al. in this respect. It has been demonstrated that some steroid 2013, Numakawa et al. 2014), thyroid hormones (Duntas metabolites long considered merely the products of steroid and Mails 2013), inflammation (Halaris 2013), immunity hormone metabolism in fact possess considerable activity in the (Pitychoutis and Papadopoulou-Daifoti 2010), melatonin CNS. For this reason we studied the steroid metabolome (Boyce and Hopwood 2013), oxytocin and vasopressin including 50 analytes in 20 men with depression, 20 men with (Scantamburlo et al. 2009, Matsuzaki et al. 2012), the anxiety and 30 healthy controls. Significant differences were renin-angiotensin-aldosterone system (Franklin et al. found not only between controls and men with either depression 2012, Murck et al. 2012), the cannabinoid system or anxiety, but also between men with depression and anxiety. (Martykánová 2010, Gorzalka and Hill 2011, Smaga et Particularly striking were those steroids until now not generally al. -

Drug Testing Program
DRUG TESTING PROGRAM Copyright © 2021 CrossFit, LLC. All Rights Reserved. CrossFit is a registered trademark ® of CrossFit, LLC. 2021 DRUG TESTING PROGRAM 2021 DRUG TESTING CONTENTS 1. DRUG-FREE COMPETITION 2. ATHLETE CONSENT 3. DRUG TESTING 4. IN-COMPETITION/OUT-OF-COMPETITION DRUG TESTING 5. REGISTERED ATHLETE TESTING POOL (OUT-OF-COMPETITION DRUG TESTING) 6. REMOVAL FROM TESTING POOL/RETIREMENT 6A. REMOVAL FROM TESTING POOL/WATCH LIST 7. TESTING POOL REQUIREMENTS FOLLOWING A SANCTION 8. DRUG TEST NOTIFICATION AND ADMINISTRATION 9. SPECIMEN ANALYSIS 10. REPORTING RESULTS 11. DRUG TESTING POLICY VIOLATIONS 12. ENFORCEMENT/SANCTIONS 13. APPEALS PROCESS 14. LEADERBOARD DISPLAY 15. EDUCATION 16. DIETARY SUPPLEMENTS 17. TRANSGENDER POLICY 18. THERAPEUTIC USE EXEMPTION APPENDIX A: 2020-2021 CROSSFIT BANNED SUBSTANCE CLASSES APPENDIX B: CROSSFIT URINE TESTING PROCEDURES - (IN-COMPETITION) APPENDIX C: TUE APPLICATION REQUIREMENTS Drug Testing Policy V4 Copyright © 2021 CrossFit, LLC. All Rights Reserved. CrossFit is a registered trademark ® of CrossFit, LLC. [ 2 ] 2021 DRUG TESTING PROGRAM 2021 DRUG TESTING 1. DRUG-FREE COMPETITION As the world’s definitive test of fitness, CrossFit Games competitions stand not only as testaments to the athletes who compete but to the training methodologies they use. In this arena, a true and honest comparison of training practices and athletic capacity is impossible without a level playing field. Therefore, the use of banned performance-enhancing substances is prohibited. Even the legal use of banned substances, such as physician-prescribed hormone replacement therapy or some over-the-counter performance-enhancing supplements, has the potential to compromise the integrity of the competition and must be disallowed. With the health, safety, and welfare of the athletes, and the integrity of our sport as top priorities, CrossFit, LLC has adopted the following Drug Testing Policy to ensure the validity of the results achieved in competition. -

Increased and Mistimed Sex Hormone Production in Night Shift Workers
Published OnlineFirst March 3, 2015; DOI: 10.1158/1055-9965.EPI-14-1271 Research Article Cancer Epidemiology, Biomarkers Increased and Mistimed Sex Hormone Production & Prevention in Night Shift Workers Kyriaki Papantoniou1,2,3,4, Oscar J. Pozo2, Ana Espinosa1,2,3,4, Josep Marcos2,3, Gemma Castano-Vinyals~ 1,2,3,4, Xavier Basagana~ 1,2,3,4, Elena Juanola Pages 5, Joan Mirabent6,7, Jordi Martín8, Patricia Such Faro9, Amparo Gasco Aparici10, Benita Middleton11, Debra J. Skene11, and Manolis Kogevinas1,2,3,4,12 Abstract Background: Night shift work has been associated with Results: Night workers had higher levels of total progestagens an increased risk for breast and prostate cancer. The effect [geometric mean ratio (GMR) 1.65; 95% confidence intervals of circadian disruption on sex steroid production is a pos- (CI), 1.17–2.32] and androgens (GMR: 1.44; 95% CI, 1.03–2.00), sible underlying mechanism, underinvestigated in hum- compared with day workers, after adjusting for potential con- ans. We have assessed daily rhythms of sex hormones founders. The increased sex hormone levels among night and melatonin in night and day shift workers of both shift workers were not related to the observed suppression of sexes. 6-sulfatoxymelatonin. Peak time of androgens was significantly Methods: We recruited 75 night and 42 day workers, ages later among night workers, compared with day workers (testos- 22 to 64 years, in different working settings. Participants terone: 12:14 hours; 10:06-14:48 vs. 08:35 hours; 06:52-10:46). collected urine samples from all voids over 24 hours on a Conclusions: We found increased levels of progestagens and working day. -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -

A10 Anabolic Steroids Hardcore Info
CONTENTS GENERAL INFORMATION 3 Anabolic steroids – What are they? 4 How do they Work? – Aromatisation 5 More molecules – More problems 6 The side effects of anabolic steroids 7 Women and anabolic steroids 8 Injecting steroids 9 Abscesses – Needle Exchanges 10 Intramuscular injection 11 Injection sites 12 Oral steroids – Cycles – Stacking 13 Diet 14 Where do steroids come from? Spotting a counterfeit 15 Drug Information – Drug dosage STEROIDS 16 Anadrol – Andriol 17 Anavar – Deca-Durabolin 18 Dynabolon – Durabolin – Dianabol 19 Esiclene – Equipoise 20 Primobolan Depot – Proviron – Primobolan orals – Pronobol 21 Sustanon – Stromba, Strombaject – Testosterone Cypionate Testosterone Enanthate 22 Testosterone Propionate – Testosterone Suspension 23 Trenbolone Acetate – Winstrol OTHER DRUGS 24 Aldactone – Arimidex 25 Clenbuterol – Cytomel 26 Ephedrine Hydrochloride – GHB 27 Growth Hormone 28 Insulin 30 Insulin-Like Growth Factor-1 – Human Chorionic Gonadotrophin 31 Tamoxifen – Nubain – Recreational Drugs 32 Steroids and the Law 34 Glossary ANABOLIC STEROIDS People use anabolic steroids for various reasons, some use them to build muscle for their job, others just want to look good and some use them to help them in sport or body building. Whatever the reason, care needs to be taken so that as little harm is done to the body as possible because despite having muscle building effects they also have serious side effects especially when used incorrectly. WHAT ARE THEY? Anabolic steroids are man made versions of the hormone testosterone. Testosterone is the chemical in men responsible for facial hair, deepening of the voice and sex organ development, basically the masculine things Steroids are in a man. used in medicine to treat anaemia, muscle weakness after These masculine effects surgery etc, vascular are called the androgenic disorders and effects of testosterone. -

2010 Prohibited List
The World Anti-Doping Code THE 2010 PROHIBITED LIST INTERNATIONAL STANDARD The official text of the Prohibited List shall be maintained by WADA and shall be published in English and French. In the event of any conflict between the English and French versions, the English version shall prevail. This List shall come into effect on 1 January 2010 The Prohibited List 2010 19 September 2009 THE 2010 PROHIBITED LIST WORLD ANTI-DOPING CODE Valid 1 January 2010 All Prohibited Substances shall be considered as “Specified Substances” except Substances in classes S1, S2.1 to S2.5, S.4.4 and S6.a, and Prohibited Methods M1, M2 and M3. SUBSTANCES AND METHODS PROHIBITED AT ALL TIMES (IN- AND OUT-OF-COMPETITION) PROHIBITED SUBSTANCES S1. ANABOLIC AGENTS Anabolic agents are prohibited. 1. Anabolic Androgenic Steroids (AAS) a. Exogenous* AAS, including: 1-androstendiol (5α-androst-1-ene-3β,17β-diol ); 1-androstendione (5α- androst-1-ene-3,17-dione); bolandiol (19-norandrostenediol); bolasterone; boldenone; boldione (androsta-1,4-diene-3,17-dione); calusterone; clostebol; danazol (17α-ethynyl-17β-hydroxyandrost-4-eno[2,3-d]isoxazole); dehydrochlormethyltestosterone (4-chloro-17β-hydroxy-17α-methylandrosta- 1,4-dien-3-one); desoxymethyltestosterone (17α-methyl-5α-androst-2-en- 17β-ol); drostanolone; ethylestrenol (19-nor-17α-pregn-4-en-17-ol); fluoxymesterone; formebolone; furazabol (17β-hydroxy-17α-methyl-5α- androstano[2,3-c]-furazan); gestrinone; 4-hydroxytestosterone (4,17β- dihydroxyandrost-4-en-3-one); mestanolone; mesterolone; metenolone; methandienone -

Drug Name Plate Number Well Location % Inhibition, Screen Axitinib 1 1 20 Gefitinib (ZD1839) 1 2 70 Sorafenib Tosylate 1 3 21 Cr
Drug Name Plate Number Well Location % Inhibition, Screen Axitinib 1 1 20 Gefitinib (ZD1839) 1 2 70 Sorafenib Tosylate 1 3 21 Crizotinib (PF-02341066) 1 4 55 Docetaxel 1 5 98 Anastrozole 1 6 25 Cladribine 1 7 23 Methotrexate 1 8 -187 Letrozole 1 9 65 Entecavir Hydrate 1 10 48 Roxadustat (FG-4592) 1 11 19 Imatinib Mesylate (STI571) 1 12 0 Sunitinib Malate 1 13 34 Vismodegib (GDC-0449) 1 14 64 Paclitaxel 1 15 89 Aprepitant 1 16 94 Decitabine 1 17 -79 Bendamustine HCl 1 18 19 Temozolomide 1 19 -111 Nepafenac 1 20 24 Nintedanib (BIBF 1120) 1 21 -43 Lapatinib (GW-572016) Ditosylate 1 22 88 Temsirolimus (CCI-779, NSC 683864) 1 23 96 Belinostat (PXD101) 1 24 46 Capecitabine 1 25 19 Bicalutamide 1 26 83 Dutasteride 1 27 68 Epirubicin HCl 1 28 -59 Tamoxifen 1 29 30 Rufinamide 1 30 96 Afatinib (BIBW2992) 1 31 -54 Lenalidomide (CC-5013) 1 32 19 Vorinostat (SAHA, MK0683) 1 33 38 Rucaparib (AG-014699,PF-01367338) phosphate1 34 14 Lenvatinib (E7080) 1 35 80 Fulvestrant 1 36 76 Melatonin 1 37 15 Etoposide 1 38 -69 Vincristine sulfate 1 39 61 Posaconazole 1 40 97 Bortezomib (PS-341) 1 41 71 Panobinostat (LBH589) 1 42 41 Entinostat (MS-275) 1 43 26 Cabozantinib (XL184, BMS-907351) 1 44 79 Valproic acid sodium salt (Sodium valproate) 1 45 7 Raltitrexed 1 46 39 Bisoprolol fumarate 1 47 -23 Raloxifene HCl 1 48 97 Agomelatine 1 49 35 Prasugrel 1 50 -24 Bosutinib (SKI-606) 1 51 85 Nilotinib (AMN-107) 1 52 99 Enzastaurin (LY317615) 1 53 -12 Everolimus (RAD001) 1 54 94 Regorafenib (BAY 73-4506) 1 55 24 Thalidomide 1 56 40 Tivozanib (AV-951) 1 57 86 Fludarabine -

Chlorpromazine(BAN, Rinn)
Carpipramine Hydrochloride/Chlorpromazine 969 2. Stubb S, et al. Fixed drug eruptions: 77 cases from 1981 to 1985. Chlorpromazine Embonate (BANM, rINNM) mazine. Photosensitivity reactions are more common Br J Dermatol 1989; 120: 583. 3. Roujeau J-C, et al. Medication use and the risk of Stevens-John- Chlorpromazine, Embonate de; Chlorpromazine Pamoate; with chlorpromazine than with other antipsychotics. son syndrome or toxic epidermal necrolysis. N Engl J Med 1995; Chlorpromazini Embonas; Embonato de clorpromazina. Haematological disorders, including haemolytic anae- 333: 1600–7. Хлорпромазина Эмбонат mia, aplastic anaemia, thrombocytopenic purpura, Porphyria. Chlormezanone has been associated with acute at- (C H ClN S) ,C H O = 1026.1. tacks of porphyria and is considered unsafe in porphyric patients. 17 19 2 2 23 16 6 eosinophilia, and a potentially fatal agranulocytosis ATC — N05AA01. have occasionally been reported; they may be manifes- Preparations ATC Vet — QN05AA01. tations of a hypersensitivity reaction. Most cases of Proprietary Preparations (details are given in Part 3) agranulocytosis have occurred within 4 to 10 weeks of Chile: Cardiosedantol; Restoril†. Chlorpromazine Hydrochloride (BANM, rINNM) starting treatment, and symptoms such as sore throat or Multi-ingredient: Chile: Adalgen†; Calmosedan; Diapam; Dioran†; Dol- Aminazine; Chloropromazyny chlorowodorek; Chlorpromazin nix; Dolonase; Dolorelax†; Fibrorelax; Mesolona†; Multisedil; Neo Butar- fever should be watched for and white cell counts insti- trol; Promidan; Sedantol; Sedilit; Silrelax†; Sin-Algin; Hong Kong: Parazone; hydrochlorid; Chlorpromazine, chlorhydrate de; Chlorpromazini S.Afr.: Myoflex. hydrochloridum; Chlorpromazino hidrochloridas; Hidrocloruro tuted should they appear. Mild leucopenia has been de clorpromazina; Klooripromatsiinihydrokloridi; Klorpromazin stated to occur in up to 30% of patients on prolonged Hidroklorür; Klórpromazin-hidroklorid; Klorpromazinhydro- high dosage.