Gc/Ms Assays for Abused Drugs in Body Fluids
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download Full Article As
Article Alkaloids Detection in Commonly Found Medicinal Plants with Marquis Reagent Daniel Alejandro Ocampo-Bustos1 and María Elena Cano-Ruiz1 1 Tecnológico de Monterrey High School, Cuernavaca, Mexico. SUMMARY identity of social groups. Many of the medicinal plants have Alkaloids are a class of nitrogenous organic their healing properties known by empirical use through time, compounds of plant origin that may have important but these medicinal plants may contain active ingredients physiological actions on humans. They include many with tested pharmacological properties. One possibility is that drugs and poisons, but some alkaloids in low doses some of the active ingredients in medicinal plants belong to the have health benefits as well. Traditional medicinal group of alkaloids, which can be determined by a colorimetric plants may contain alkaloids as active ingredients, chemical reaction with the Marquis reagent. The reagent is but this is not well-understood. The Marquis reagent dripped onto the substance being tested, and if an alkaloid exists as a simple qualitative colorimetric method is present, a color change appears (5). The Marquis reagent to determine the presence of alkaloids in medicinal is traditionally composed of a mixture of formaldehyde and plants. The Marquis reagent test was assayed in concentrated sulfuric acid. medicinal plants by first optimizing the formulation Originally, the Marquis reagent was used for testing of the reagent using poppy seeds and lavender as many different alkaloids, and the results from those studies the positive and negative controls. Then using the were the base for developing the color scales that are optimized formulation of Marquis reagent in the extracts of 11 medicinal plants with known claims of used as a reference to determine the specific alkaloid health benefits. -

Classification of Medicinal Drugs and Driving: Co-Ordination and Synthesis Report
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Due date of deliverable: 21.07.2011 Actual submission date: 21.07.2011 Revision date: 21.07.2011 Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UVA Revision 0.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public PP Restricted to other programme participants (including the Commission x Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) DRUID 6th Framework Programme Deliverable D.4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Page 1 of 243 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Authors Trinidad Gómez-Talegón, Inmaculada Fierro, M. Carmen Del Río, F. Javier Álvarez (UVa, University of Valladolid, Spain) Partners - Silvia Ravera, Susana Monteiro, Han de Gier (RUGPha, University of Groningen, the Netherlands) - Gertrude Van der Linden, Sara-Ann Legrand, Kristof Pil, Alain Verstraete (UGent, Ghent University, Belgium) - Michel Mallaret, Charles Mercier-Guyon, Isabelle Mercier-Guyon (UGren, University of Grenoble, Centre Regional de Pharmacovigilance, France) - Katerina Touliou (CERT-HIT, Centre for Research and Technology Hellas, Greece) - Michael Hei βing (BASt, Bundesanstalt für Straßenwesen, Germany). -

(12) United States Patent (10) Patent No.: US 8,980,319 B2 Park Et Al
US00898O319B2 (12) United States Patent (10) Patent No.: US 8,980,319 B2 Park et al. (45) Date of Patent: *Mar. 17, 2015 (54) METHODS OF PRODUCING STABILIZED A613 L/445 (2006.01) SOLID DOSAGE PHARMACEUTICAL A613 L/47 (2006.01) COMPOSITIONS CONTAINING A6II 45/06 (2006.01) MORPHINANS A63/67 (2006.01) (52) U.S. Cl. (71) Applicant: Mallinckrodt LLC, Hazelwood, MO CPC ............. A6 IK3I/485 (2013.01); A61 K9/1652 (US) (2013.01); A61 K9/2031 (2013.01); A61 K 9/2081 (2013.01); A61 K9/2086 (2013.01); (72) Inventors: Jae Han Park, Olivette, MO (US); A6IK9/2095 (2013.01); A61 K9/5042 Tiffani Eisenhauer, Columbia, IL (US); (2013.01); A61 K3I/4355 (2013.01); A61 K Spainty,S.Isna Gupta, F11llsborough, 31/4375A6 (2013.01); IK3I/445 gets (2013.01); it' A6 (2013.01); IK3I/47 Stephen Overholt, Middlesex, NJ (US) (2013.01); A61K 45/06 (2013.01); A61 K 9/2013 (2013.01); A61 K9/209 (2013.01); (73) Assignee: Mallinckrodt LLC, Hazelwood, MO A6 IK3I/167 (2013.01) (US) USPC ........... 424/472: 424/465; 424/468; 424/490; c - r - 514/282; 514/289 (*) Notice: Subject to any disclaimer, the term of this (58) Field of Classification Search patent is extended or adjusted under 35 N U.S.C. 154(b)b) by 0 daysyS. Seeone application file for complete search history. This patent is Subject to a terminal dis claimer. (56) References Cited (21) Appl. No.: 14/092.375 U.S. PATENT DOCUMENTS (22) Filed: Nov. 27, 2013 2008, 0026052 A1 ck 1/2008 Schoenhard ................. -

Chlorpromazine(BAN, Rinn)
Carpipramine Hydrochloride/Chlorpromazine 969 2. Stubb S, et al. Fixed drug eruptions: 77 cases from 1981 to 1985. Chlorpromazine Embonate (BANM, rINNM) mazine. Photosensitivity reactions are more common Br J Dermatol 1989; 120: 583. 3. Roujeau J-C, et al. Medication use and the risk of Stevens-John- Chlorpromazine, Embonate de; Chlorpromazine Pamoate; with chlorpromazine than with other antipsychotics. son syndrome or toxic epidermal necrolysis. N Engl J Med 1995; Chlorpromazini Embonas; Embonato de clorpromazina. Haematological disorders, including haemolytic anae- 333: 1600–7. Хлорпромазина Эмбонат mia, aplastic anaemia, thrombocytopenic purpura, Porphyria. Chlormezanone has been associated with acute at- (C H ClN S) ,C H O = 1026.1. tacks of porphyria and is considered unsafe in porphyric patients. 17 19 2 2 23 16 6 eosinophilia, and a potentially fatal agranulocytosis ATC — N05AA01. have occasionally been reported; they may be manifes- Preparations ATC Vet — QN05AA01. tations of a hypersensitivity reaction. Most cases of Proprietary Preparations (details are given in Part 3) agranulocytosis have occurred within 4 to 10 weeks of Chile: Cardiosedantol; Restoril†. Chlorpromazine Hydrochloride (BANM, rINNM) starting treatment, and symptoms such as sore throat or Multi-ingredient: Chile: Adalgen†; Calmosedan; Diapam; Dioran†; Dol- Aminazine; Chloropromazyny chlorowodorek; Chlorpromazin nix; Dolonase; Dolorelax†; Fibrorelax; Mesolona†; Multisedil; Neo Butar- fever should be watched for and white cell counts insti- trol; Promidan; Sedantol; Sedilit; Silrelax†; Sin-Algin; Hong Kong: Parazone; hydrochlorid; Chlorpromazine, chlorhydrate de; Chlorpromazini S.Afr.: Myoflex. hydrochloridum; Chlorpromazino hidrochloridas; Hidrocloruro tuted should they appear. Mild leucopenia has been de clorpromazina; Klooripromatsiinihydrokloridi; Klorpromazin stated to occur in up to 30% of patients on prolonged Hidroklorür; Klórpromazin-hidroklorid; Klorpromazinhydro- high dosage. -
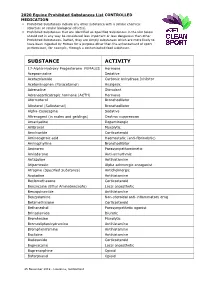
2020 Equine Prohibited Substances List CONTROLLED MEDICATION
2020 Equine Prohibited Substances List CONTROLLED MEDICATION . Prohibited Substances include any other substance with a similar chemical structure or similar biological effect(s). Prohibited Substances that are identified as Specified Substances in the List below should not in any way be considered less important or less dangerous than other Prohibited Substances. Rather, they are simply substances which are more likely to have been ingested by Horses for a purpose other than the enhancement of sport performance, for example, through a contaminated food substance. SUBSTANCE ACTIVITY 17-Alpha-Hydroxy Progesterone FEMALES Hormone Acepromazine Sedative Acetazolamide Carbonic Anhydrase Inhibitor Acetominophen (Paracetamol) Analgesic Adrenaline Stimulant Adrenocorticotropic hormone (ACTH) Hormone Aformoterol Bronchodilator Albuterol (Salbutamol) Bronchodilator Alpha-Casozepine Sedative Altrenogest (in males and geldings) Oestrus suppression Amantadine Dopaminergic Ambroxol Mucolytic Amcinonide Corticosteroid Aminocaproic acid Haemostatic (anti-fibrinolytic) Aminophylline Bronchodilator Aminorex Parasympathomimetic Amiodarone Anti-arrhythmic Antazoline Antihistamine Atipamezole Alpha adrenergic antagonist Atropine (Specified Substance) Anticholinergic Azatadine Antihistamine Beclomethasone Corticosteroid Benzocaine (Ethyl Aminobenzoate) Local anaesthetic Benzquinamide Antihistamine Benzydamine Non-steroidal anti-inflammatory drug Betamethasone Corticosteroid Bethanechol Parasympathetic agonist Brinzolamide Diuretic Bromhexine Mucolytic Bromodiphenhydramine -

Dr. Duke's Phytochemical and Ethnobotanical Databases Chemicals Found in Papaver Somniferum
Dr. Duke's Phytochemical and Ethnobotanical Databases Chemicals found in Papaver somniferum Activities Count Chemical Plant Part Low PPM High PPM StdDev Refernce Citation 0 (+)-LAUDANIDINE Fruit -- 0 (+)-RETICULINE Fruit -- 0 (+)-RETICULINE Latex Exudate -- 0 (-)-ALPHA-NARCOTINE Inflorescence -- 0 (-)-NARCOTOLINE Inflorescence -- 0 (-)-SCOULERINE Latex Exudate -- 0 (-)-SCOULERINE Plant -- 0 10-HYDROXYCODEINE Latex Exudate -- 0 10-NONACOSANOL Latex Exudate Chemical Constituents of Oriental Herbs (3 diff. books) 0 13-OXOCRYPTOPINE Plant -- 0 16-HYDROXYTHEBAINE Plant -- 0 20-HYDROXY- Fruit 36.0 -- TRICOSANYLCYCLOHEXA NE 0 4-HYDROXY-BENZOIC- Pericarp -- ACID 0 4-METHYL-NONACOSANE Fruit 3.2 -- 0 5'-O- Plant -- DEMETHYLNARCOTINE 0 5-HYDROXY-3,7- Latex Exudate -- DIMETHOXYPHENANTHRE NE 0 6- Plant -- ACTEONLYDIHYDROSANG UINARINE 0 6-METHYL-CODEINE Plant Father Nature's Farmacy: The aggregate of all these three-letter citations. 0 6-METHYL-CODEINE Fruit -- 0 ACONITASE Latex Exudate -- 32 AESCULETIN Pericarp -- 3 ALANINE Seed 11780.0 12637.0 0.5273634907250652 -- Activities Count Chemical Plant Part Low PPM High PPM StdDev Refernce Citation 0 ALKALOIDS Latex Exudate 50000.0 250000.0 ANON. 1948-1976. The Wealth of India raw materials. Publications and Information Directorate, CSIR, New Delhi. 11 volumes. 5 ALLOCRYPTOPINE Plant Father Nature's Farmacy: The aggregate of all these three-letter citations. 15 ALPHA-LINOLENIC-ACID Seed 1400.0 5564.0 -0.22115561650586155 -- 2 ALPHA-NARCOTINE Plant Jeffery B. Harborne and H. Baxter, eds. 1983. Phytochemical Dictionary. A Handbook of Bioactive Compounds from Plants. Taylor & Frost, London. 791 pp. 17 APOMORPHINE Plant Father Nature's Farmacy: The aggregate of all these three-letter citations. 0 APOREINE Fruit -- 0 ARABINOSE Fruit ANON. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Zine and Some Related Phenothiazines in Reducing the Rigidity of the Decerebrate Cat and in Some Other Central Actions by Elizabeth M
Br. J. Pharmac. Chemother. (1967), 29, 400-416. A COMPARISON OF THE EFFECTS OF CHLORPROMA- ZINE AND SOME RELATED PHENOTHIAZINES IN REDUCING THE RIGIDITY OF THE DECEREBRATE CAT AND IN SOME OTHER CENTRAL ACTIONS BY ELIZABETH M. KEARY AND D. R. MAXWELL From the Research Laboratories, May & Baker Ltd., Dagenham, Essex (Received March 2, 1967) One of the many interesting properties of chlorpromazine is its ability to reduce skeletal muscle tone in experimental animals (Dasgupta & Werner, 1955; Sheatz, 1955). Henatsch & Ingvar (1956) and Busch, Henatsch & Schulte (1960) have shown that small doses of chlorpromazine abolish the rigidity of the intercollicular decerebrate cat and reduce the discharge of -y-motoneurones. These authors also found that much larger doses of chlorpromazine were required to depress the rigidity of the decerebrate cat prepared by the ischaemic method of Pollock & Davis (1930). It was suggested that chlorpromazine had a selective depressant effect on the y- as opposed to the a-moto- neurones at a supraspinal level. Chlorpromazine has been reputed to be of some value in reducing the muscle tonus in patients with spasticity (Basmajian & Szatmari, 1955). Although the actions of chlorpromazine in reducing skeletal muscle tone and in affecting spinal reflexes have been extensively studied, little is known about the activity of related phenothiazine derivatives in this context. It thus appeared interesting to compare chlorpromazine with some related phenothiazine derivatives for their ability to reduce rigidity of the intercollicular decerebrate cat with the object of seeing to what extent there was correlation between activity in reducing skeletal muscle tone and other pharmacological actions. -

Screening of 300 Drugs in Blood Utilizing Second Generation
Forensic Screening of 300 Drugs in Blood Utilizing Exactive Plus High-Resolution Accurate Mass Spectrometer and ExactFinder Software Kristine Van Natta, Marta Kozak, Xiang He Forensic Toxicology use Only Drugs analyzed Compound Compound Compound Atazanavir Efavirenz Pyrilamine Chlorpropamide Haloperidol Tolbutamide 1-(3-Chlorophenyl)piperazine Des(2-hydroxyethyl)opipramol Pentazocine Atenolol EMDP Quinidine Chlorprothixene Hydrocodone Tramadol 10-hydroxycarbazepine Desalkylflurazepam Perimetazine Atropine Ephedrine Quinine Cilazapril Hydromorphone Trazodone 5-(p-Methylphenyl)-5-phenylhydantoin Desipramine Phenacetin Benperidol Escitalopram Quinupramine Cinchonine Hydroquinine Triazolam 6-Acetylcodeine Desmethylcitalopram Phenazone Benzoylecgonine Esmolol Ranitidine Cinnarizine Hydroxychloroquine Trifluoperazine Bepridil Estazolam Reserpine 6-Monoacetylmorphine Desmethylcitalopram Phencyclidine Cisapride HydroxyItraconazole Trifluperidol Betaxolol Ethyl Loflazepate Risperidone 7(2,3dihydroxypropyl)Theophylline Desmethylclozapine Phenylbutazone Clenbuterol Hydroxyzine Triflupromazine Bezafibrate Ethylamphetamine Ritonavir 7-Aminoclonazepam Desmethyldoxepin Pholcodine Clobazam Ibogaine Trihexyphenidyl Biperiden Etifoxine Ropivacaine 7-Aminoflunitrazepam Desmethylmirtazapine Pimozide Clofibrate Imatinib Trimeprazine Bisoprolol Etodolac Rufinamide 9-hydroxy-risperidone Desmethylnefopam Pindolol Clomethiazole Imipramine Trimetazidine Bromazepam Felbamate Secobarbital Clomipramine Indalpine Trimethoprim Acepromazine Desmethyltramadol Pipamperone -

Drug and Medication Classification Schedule
KENTUCKY HORSE RACING COMMISSION UNIFORM DRUG, MEDICATION, AND SUBSTANCE CLASSIFICATION SCHEDULE KHRC 8-020-1 (11/2018) Class A drugs, medications, and substances are those (1) that have the highest potential to influence performance in the equine athlete, regardless of their approval by the United States Food and Drug Administration, or (2) that lack approval by the United States Food and Drug Administration but have pharmacologic effects similar to certain Class B drugs, medications, or substances that are approved by the United States Food and Drug Administration. Acecarbromal Bolasterone Cimaterol Divalproex Fluanisone Acetophenazine Boldione Citalopram Dixyrazine Fludiazepam Adinazolam Brimondine Cllibucaine Donepezil Flunitrazepam Alcuronium Bromazepam Clobazam Dopamine Fluopromazine Alfentanil Bromfenac Clocapramine Doxacurium Fluoresone Almotriptan Bromisovalum Clomethiazole Doxapram Fluoxetine Alphaprodine Bromocriptine Clomipramine Doxazosin Flupenthixol Alpidem Bromperidol Clonazepam Doxefazepam Flupirtine Alprazolam Brotizolam Clorazepate Doxepin Flurazepam Alprenolol Bufexamac Clormecaine Droperidol Fluspirilene Althesin Bupivacaine Clostebol Duloxetine Flutoprazepam Aminorex Buprenorphine Clothiapine Eletriptan Fluvoxamine Amisulpride Buspirone Clotiazepam Enalapril Formebolone Amitriptyline Bupropion Cloxazolam Enciprazine Fosinopril Amobarbital Butabartital Clozapine Endorphins Furzabol Amoxapine Butacaine Cobratoxin Enkephalins Galantamine Amperozide Butalbital Cocaine Ephedrine Gallamine Amphetamine Butanilicaine Codeine -

This List Was Originally Published in Virginia Register Volume 8, Issue 18
In accordance with 6 VAC 40-30, the Regulations for the Approval of Field Tests for Detection of Drugs, and under the authority of the Code of Virginia, the following field tests for detection of drugs are approved field tests: O D V INCORPORATED 13386 INTERNATIONAL PARKWAY JACKSONVILLE, FLORIDA 32218-2383 ODV NarcoPouch Drug or Drug Type: Manufacturer’s Field Test: Heroin 902 – Marquis Reagent Amphetamine 902 – Marquis Reagent Methamphetamine 902 – Marquis Reagent 3,4–Methylenedioxymethamphetamine (MDMA) 902 – Marquis Reagent Cocaine Hydrochloride 904 or 904B – Cocaine HCl and Base Reagent Cocaine Base 904 or 904B – Cocaine HCl and Base Reagent Barbiturates 905 – Dille-Koppanyi Reagent Lysergic Acid Diethylamide (LSD) 907 – Ehrlich’s (Modified) Reagent Marijuana 908 – Duquenois – Levine Reagent Hashish Oil 908 – Duquenois – Levine Reagent Marijuana 909 – K N Reagent Hashish Oil 909 – K N Reagent Phencyclidine (PCP) 914 – PCP Methaqualone Reagent Heroin 922 – Opiates Reagent Methamphetamine 923 – Methamphetamine/Ecstasy Reagent 3,4–Methylenedioxymethamphetamine (MDMA) 923 – Methamphetamine/Ecstasy Reagent Heroin 924 – Mecke’s (Modified) Reagent Diazepam 925 – Valium/Ketamine Reagent Ketamine 925 – Valium/Ketamine Reagent Ephedrine 927 – Ephedrine Reagent gamma – Hydroxybutyrate (GHB) 928 – GHB Reagent ODV NarcoTest Drug or Drug Type: Manufacturer’s Field Test: Heroin 7602 – Marquis Reagent Amphetamine 7602 – Marquis Reagent Methamphetamine 7602 – Marquis Reagent 3,4–Methylenedioxymethamphetamine (MDMA) 7602 – Marquis Reagent Barbiturates -

Thesis-1982D-K435m.Pdf
METAL ION - DRUG INTERACTIONS IN SOLUTIONS By NITAYA KETKEAW "' Bachelor of Science Chiengmai University Chiengmai, Thailand 1975 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillments of the requirements for the Degree of DOCTOR OF PHILOSOPHY December, 1982 l~1<:.~is \q 1i ~D K l.~:lj M ~·IV' ----- METAL IN SOLUTIONS Thesis Approved: Dean of the Graduate College ii 1155645 ,. ACKNOWLEDGMENTS I wish first to express my gratitude to Dr. Neil Purdie for his patience, understanding and invaluable guidance through out this study, and for his assistance in the preparation of this manuscript. Appreciation is expressed to Dr. Larry E. Halliburton for the use of ESR facilities and his assistance in obtaining the ESR spectra, and to Dr. Tom E. Moore, Dr. Elizabeth M. Holt, Dr. J. Paul Devlin and Dr. Liao Ta-Hsiu for serving on the committee. Special thanks are extended to my fellow graduate students and to the faculty and staff of the Chemistry Department for their encouragement. I would also like to express gratitude to my parents and my husband for their patience and unfailing encouragement throughout the long years. iii TABLE OF CONTENTS Chapter Page I. INTRODUCTION • 1 Statement of the Problem 8 II. BACKGROUND AND THEORY 10 Circular Dichroism . • • 10 Origin of Light Polarization . • • • 10 Electron Spin Resonance . • • • 19 Hyperfine Structure .• . .. • 24 g - Factor • • • . • . • . • • • 30 Interpretation of ESR Spectra 31 III. EXPERIMENTAL . 33 Instrumental . • . • • • . • 33 Chemicals . • • . • • • • . • . 35 Experimental Procudures . • • • • • 36 CD and UV-Visible Measurements • 36 Acid and Base Titrations of Morphine in Aqueous Solution . • • • . • 36 Metal Ion - Drug Interactions in Aqueous Solution • • • 36 Reactions of Drugs with Con- centrated Sulfuric Acid • 37 Pseudomorphine Solutions 37 ESR Measurements • .