178CSR1 Title 178 Legislative Rule West Virginia Racing Commission
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 De Juan Et Al
US 200601 10428A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 de Juan et al. (43) Pub. Date: May 25, 2006 (54) METHODS AND DEVICES FOR THE Publication Classification TREATMENT OF OCULAR CONDITIONS (51) Int. Cl. (76) Inventors: Eugene de Juan, LaCanada, CA (US); A6F 2/00 (2006.01) Signe E. Varner, Los Angeles, CA (52) U.S. Cl. .............................................................. 424/427 (US); Laurie R. Lawin, New Brighton, MN (US) (57) ABSTRACT Correspondence Address: Featured is a method for instilling one or more bioactive SCOTT PRIBNOW agents into ocular tissue within an eye of a patient for the Kagan Binder, PLLC treatment of an ocular condition, the method comprising Suite 200 concurrently using at least two of the following bioactive 221 Main Street North agent delivery methods (A)-(C): Stillwater, MN 55082 (US) (A) implanting a Sustained release delivery device com (21) Appl. No.: 11/175,850 prising one or more bioactive agents in a posterior region of the eye so that it delivers the one or more (22) Filed: Jul. 5, 2005 bioactive agents into the vitreous humor of the eye; (B) instilling (e.g., injecting or implanting) one or more Related U.S. Application Data bioactive agents Subretinally; and (60) Provisional application No. 60/585,236, filed on Jul. (C) instilling (e.g., injecting or delivering by ocular ion 2, 2004. Provisional application No. 60/669,701, filed tophoresis) one or more bioactive agents into the Vit on Apr. 8, 2005. reous humor of the eye. Patent Application Publication May 25, 2006 Sheet 1 of 22 US 2006/0110428A1 R 2 2 C.6 Fig. -

Classification of Medicinal Drugs and Driving: Co-Ordination and Synthesis Report
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Due date of deliverable: 21.07.2011 Actual submission date: 21.07.2011 Revision date: 21.07.2011 Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UVA Revision 0.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public PP Restricted to other programme participants (including the Commission x Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) DRUID 6th Framework Programme Deliverable D.4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Page 1 of 243 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Authors Trinidad Gómez-Talegón, Inmaculada Fierro, M. Carmen Del Río, F. Javier Álvarez (UVa, University of Valladolid, Spain) Partners - Silvia Ravera, Susana Monteiro, Han de Gier (RUGPha, University of Groningen, the Netherlands) - Gertrude Van der Linden, Sara-Ann Legrand, Kristof Pil, Alain Verstraete (UGent, Ghent University, Belgium) - Michel Mallaret, Charles Mercier-Guyon, Isabelle Mercier-Guyon (UGren, University of Grenoble, Centre Regional de Pharmacovigilance, France) - Katerina Touliou (CERT-HIT, Centre for Research and Technology Hellas, Greece) - Michael Hei βing (BASt, Bundesanstalt für Straßenwesen, Germany). -

Thoroughbred Racing
178CSR1 Title 178 Legislative Rule West Virginia Racing Commission Series 1 Thoroughbred Racing Effective: July 9, 2014 West Virginia Racing Commission 900 Pennsylvania Avenue Suite 533 Charleston WV 25302 305.558.2150 Fax 304.558.6319 Web Site: www.racing.wv.gov 178CSR1 Table of Contents SERIES 1 THOROUGHBRED RACING ____________________________________________________________________ 1 §178-1-1. General. ____________________________________________________________________________________ 1 PART 1. DEFINITIONS. _________________________________________________________________________________ 1 §178-1-2. Definitions. _________________________________________________________________________________ 1 PART 2. GENERAL AUTHORITY. ________________________________________________________________________ 9 §178-1-3. General Authority of the Racing Commission. ______________________________________________________ 9 §178-1-4. Power Of Entry. ______________________________________________________________________________ 9 §178-1-5. Racing Commission personnel. __________________________________________________________________ 9 §178-1-6. Ejection/Exclusion. __________________________________________________________________________ 12 PART 3. RACING OFFICIALS. __________________________________________________________________________ 12 §178-1-7. General Provisions. __________________________________________________________________________ 12 §178-1-8. Stewards. __________________________________________________________________________________ 14 -

Customs Tariff - Schedule
CUSTOMS TARIFF - SCHEDULE 99 - i Chapter 99 SPECIAL CLASSIFICATION PROVISIONS - COMMERCIAL Notes. 1. The provisions of this Chapter are not subject to the rule of specificity in General Interpretative Rule 3 (a). 2. Goods which may be classified under the provisions of Chapter 99, if also eligible for classification under the provisions of Chapter 98, shall be classified in Chapter 98. 3. Goods may be classified under a tariff item in this Chapter and be entitled to the Most-Favoured-Nation Tariff or a preferential tariff rate of customs duty under this Chapter that applies to those goods according to the tariff treatment applicable to their country of origin only after classification under a tariff item in Chapters 1 to 97 has been determined and the conditions of any Chapter 99 provision and any applicable regulations or orders in relation thereto have been met. 4. The words and expressions used in this Chapter have the same meaning as in Chapters 1 to 97. Issued January 1, 2020 99 - 1 CUSTOMS TARIFF - SCHEDULE Tariff Unit of MFN Applicable SS Description of Goods Item Meas. Tariff Preferential Tariffs 9901.00.00 Articles and materials for use in the manufacture or repair of the Free CCCT, LDCT, GPT, UST, following to be employed in commercial fishing or the commercial MT, MUST, CIAT, CT, harvesting of marine plants: CRT, IT, NT, SLT, PT, COLT, JT, PAT, HNT, Artificial bait; KRT, CEUT, UAT, CPTPT: Free Carapace measures; Cordage, fishing lines (including marlines), rope and twine, of a circumference not exceeding 38 mm; Devices for keeping nets open; Fish hooks; Fishing nets and netting; Jiggers; Line floats; Lobster traps; Lures; Marker buoys of any material excluding wood; Net floats; Scallop drag nets; Spat collectors and collector holders; Swivels. -

Systematic Review of the Effect of Intravenous Lipid Emulsion Therapy for Non-Local Anesthetics Toxicity
Clinical Toxicology ISSN: 1556-3650 (Print) 1556-9519 (Online) Journal homepage: http://www.tandfonline.com/loi/ictx20 Systematic review of the effect of intravenous lipid emulsion therapy for non-local anesthetics toxicity Michael Levine, Robert S. Hoffman, Valéry Lavergne, Christine M. Stork, Andis Graudins, Ryan Chuang, Samuel J. Stellpflug, Martin Morris, Andrea Miller-Nesbitt, Sophie Gosselin & for the Lipid Emulsion Workgroup* To cite this article: Michael Levine, Robert S. Hoffman, Valéry Lavergne, Christine M. Stork, Andis Graudins, Ryan Chuang, Samuel J. Stellpflug, Martin Morris, Andrea Miller-Nesbitt, Sophie Gosselin & for the Lipid Emulsion Workgroup* (2016) Systematic review of the effect of intravenous lipid emulsion therapy for non-local anesthetics toxicity, Clinical Toxicology, 54:3, 194-221, DOI: 10.3109/15563650.2015.1126286 To link to this article: http://dx.doi.org/10.3109/15563650.2015.1126286 Published online: 06 Feb 2016. Submit your article to this journal Article views: 692 View related articles View Crossmark data Citing articles: 2 View citing articles Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=ictx20 Download by: [UPSTATE Medical University Health Sciences Library] Date: 03 August 2016, At: 08:19 CLINICAL TOXICOLOGY, 2016 VOL. 54, NO. 3, 194–221 http://dx.doi.org/10.3109/15563650.2015.1126286 REVIEW Systematic review of the effect of intravenous lipid emulsion therapy for non-local anesthetics toxicity Michael Levinea, Robert S. Hoffmanb, Vale´ry Lavergnec, Christine M. Storkd, Andis Graudinse, Ryan Chuangf, Samuel J. Stellpflugg, Martin Morrish, Andrea Miller-Nesbitth, Sophie Gosselini and for the Lipid Emulsion Workgroup* aDepartment of Emergency Medicine, Section of Medical Toxicology, University of Southern California, Los Angeles, CA, USA; bDivision of Medical Toxicology, Ronald O. -

(12) United States Patent (10) Patent No.: US 9,314.465 B2 Brew Et Al
US009314465B2 (12) United States Patent (10) Patent No.: US 9,314.465 B2 Brew et al. (45) Date of Patent: *Apr. 19, 2016 (54) DRUG COMBINATIONS AND USES IN 2008.0003280 A1 1/2008 Levine et al. ................. 424/456 TREATING A COUGHING CONDITION 2008/O176955 A1 7/2008 Hecket al. 2008, 0220078 A1 9, 2008 Morton et al. (71) Applicant: Infirst Healthcare Limited 2009, O136427 A1 5/2009 Croft et al. 2009, O220594 A1 9, 2009 Field (72) Inventors: John Brew, London (GB); Robin Mark 2012/O128738 A1 5, 2012 Brew et al. Bannister, London (GB) 2012fO252824 A1 10/2012 Brew et al. (73) Assignee: Infirst Healthcare Limited, London FOREIGN PATENT DOCUMENTS (GB) CN 1593451 3, 2005 CN 101024.014 A 8, 2007 (*) Notice: Subject to any disclaimer, the term of this CN 101112383 B 5, 2010 patent is extended or adjusted under 35 DE 4420708 A1 12, 1995 U.S.C. 154(b) by 0 days. EP 2050435 B1 4/2009 GB 2114001 A 8, 1983 This patent is Subject to a terminal dis GB 2284761 A 6, 1995 claimer. GB 2424.185 B 9, 2006 GB 2442828 A 4/2008 JP 62-249924 A 10, 1987 (21) Appl. No.: 14/287,014 JP H1O-316568 A 12/1998 JP 2001-518928 A 10, 2001 (22) Filed: May 24, 2014 JP 200219.3839. A T 2002 JP 2003-012514 A 1, 2003 (65) Prior Publication Data JP 20030552.58 A 2, 2003 JP 2003128549 A 5, 2003 US 2014/O256750 A1 Sep. 11, 2014 JP 2003-321357 A 11, 2003 JP 2005-516917 A 6, 2005 JP 2008O31146 A 2, 2008 Related U.S. -

UNIVERSITE DE NANTES Thomas Gelineau
UNIVERSITE DE NANTES __________ FACULTE DE MEDECINE __________ Année 2011 N° 139 THESE pour le DIPLÔME D’ÉTAT DE DOCTEUR EN MÉDECINE DES de médecine générale par Thomas Gelineau né le 29 janvier 1983 à Cholet __________ Présentée et soutenue publiquement le 06/12/2011 __________ LE RHUME DE L'ENFANT ET SON TRAITEMENT: DECISION PARTAGEE AVEC LES PARENTS D'APRES UN QUESTIONNAIRE __________ Président : Monsieur le Professeur Olivier MALARD Directeur de thèse : Madame le Professeur Jacqueline LACAILLE 1 Table des matières IIntroduction................................................................................................................ 7 IIDéfinition, état des connaissances...........................................................................8 1Le rhume.......................................................................................................................................8 APhysiopathologie............................................................................................................................. 8 aL'origine virale.................................................................................................................................... 8 bLa saisonnalité................................................................................................................................... 8 cL'âge de survenue.............................................................................................................................. 9 dLe sexe.................................................................................................................................................. -

Chlorpromazine(BAN, Rinn)
Carpipramine Hydrochloride/Chlorpromazine 969 2. Stubb S, et al. Fixed drug eruptions: 77 cases from 1981 to 1985. Chlorpromazine Embonate (BANM, rINNM) mazine. Photosensitivity reactions are more common Br J Dermatol 1989; 120: 583. 3. Roujeau J-C, et al. Medication use and the risk of Stevens-John- Chlorpromazine, Embonate de; Chlorpromazine Pamoate; with chlorpromazine than with other antipsychotics. son syndrome or toxic epidermal necrolysis. N Engl J Med 1995; Chlorpromazini Embonas; Embonato de clorpromazina. Haematological disorders, including haemolytic anae- 333: 1600–7. Хлорпромазина Эмбонат mia, aplastic anaemia, thrombocytopenic purpura, Porphyria. Chlormezanone has been associated with acute at- (C H ClN S) ,C H O = 1026.1. tacks of porphyria and is considered unsafe in porphyric patients. 17 19 2 2 23 16 6 eosinophilia, and a potentially fatal agranulocytosis ATC — N05AA01. have occasionally been reported; they may be manifes- Preparations ATC Vet — QN05AA01. tations of a hypersensitivity reaction. Most cases of Proprietary Preparations (details are given in Part 3) agranulocytosis have occurred within 4 to 10 weeks of Chile: Cardiosedantol; Restoril†. Chlorpromazine Hydrochloride (BANM, rINNM) starting treatment, and symptoms such as sore throat or Multi-ingredient: Chile: Adalgen†; Calmosedan; Diapam; Dioran†; Dol- Aminazine; Chloropromazyny chlorowodorek; Chlorpromazin nix; Dolonase; Dolorelax†; Fibrorelax; Mesolona†; Multisedil; Neo Butar- fever should be watched for and white cell counts insti- trol; Promidan; Sedantol; Sedilit; Silrelax†; Sin-Algin; Hong Kong: Parazone; hydrochlorid; Chlorpromazine, chlorhydrate de; Chlorpromazini S.Afr.: Myoflex. hydrochloridum; Chlorpromazino hidrochloridas; Hidrocloruro tuted should they appear. Mild leucopenia has been de clorpromazina; Klooripromatsiinihydrokloridi; Klorpromazin stated to occur in up to 30% of patients on prolonged Hidroklorür; Klórpromazin-hidroklorid; Klorpromazinhydro- high dosage. -
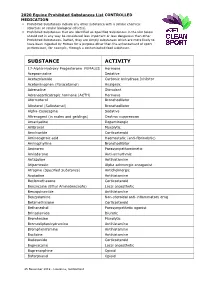
2020 Equine Prohibited Substances List CONTROLLED MEDICATION
2020 Equine Prohibited Substances List CONTROLLED MEDICATION . Prohibited Substances include any other substance with a similar chemical structure or similar biological effect(s). Prohibited Substances that are identified as Specified Substances in the List below should not in any way be considered less important or less dangerous than other Prohibited Substances. Rather, they are simply substances which are more likely to have been ingested by Horses for a purpose other than the enhancement of sport performance, for example, through a contaminated food substance. SUBSTANCE ACTIVITY 17-Alpha-Hydroxy Progesterone FEMALES Hormone Acepromazine Sedative Acetazolamide Carbonic Anhydrase Inhibitor Acetominophen (Paracetamol) Analgesic Adrenaline Stimulant Adrenocorticotropic hormone (ACTH) Hormone Aformoterol Bronchodilator Albuterol (Salbutamol) Bronchodilator Alpha-Casozepine Sedative Altrenogest (in males and geldings) Oestrus suppression Amantadine Dopaminergic Ambroxol Mucolytic Amcinonide Corticosteroid Aminocaproic acid Haemostatic (anti-fibrinolytic) Aminophylline Bronchodilator Aminorex Parasympathomimetic Amiodarone Anti-arrhythmic Antazoline Antihistamine Atipamezole Alpha adrenergic antagonist Atropine (Specified Substance) Anticholinergic Azatadine Antihistamine Beclomethasone Corticosteroid Benzocaine (Ethyl Aminobenzoate) Local anaesthetic Benzquinamide Antihistamine Benzydamine Non-steroidal anti-inflammatory drug Betamethasone Corticosteroid Bethanechol Parasympathetic agonist Brinzolamide Diuretic Bromhexine Mucolytic Bromodiphenhydramine -

Prohibited Substances List
Prohibited Substances List This is the Equine Prohibited Substances List that was voted in at the FEI General Assembly in November 2009 alongside the new Equine Anti-Doping and Controlled Medication Regulations(EADCMR). Neither the List nor the EADCM Regulations are in current usage. Both come into effect on 1 January 2010. The current list of FEI prohibited substances remains in effect until 31 December 2009 and can be found at Annex II Vet Regs (11th edition) Changes in this List : Shaded row means that either removed or allowed at certain limits only SUBSTANCE ACTIVITY Banned Substances 1 Acebutolol Beta blocker 2 Acefylline Bronchodilator 3 Acemetacin NSAID 4 Acenocoumarol Anticoagulant 5 Acetanilid Analgesic/anti-pyretic 6 Acetohexamide Pancreatic stimulant 7 Acetominophen (Paracetamol) Analgesic/anti-pyretic 8 Acetophenazine Antipsychotic 9 Acetylmorphine Narcotic 10 Adinazolam Anxiolytic 11 Adiphenine Anti-spasmodic 12 Adrafinil Stimulant 13 Adrenaline Stimulant 14 Adrenochrome Haemostatic 15 Alclofenac NSAID 16 Alcuronium Muscle relaxant 17 Aldosterone Hormone 18 Alfentanil Narcotic 19 Allopurinol Xanthine oxidase inhibitor (anti-hyperuricaemia) 20 Almotriptan 5 HT agonist (anti-migraine) 21 Alphadolone acetate Neurosteriod 22 Alphaprodine Opiod analgesic 23 Alpidem Anxiolytic 24 Alprazolam Anxiolytic 25 Alprenolol Beta blocker 26 Althesin IV anaesthetic 27 Althiazide Diuretic 28 Altrenogest (in males and gelidngs) Oestrus suppression 29 Alverine Antispasmodic 30 Amantadine Dopaminergic 31 Ambenonium Cholinesterase inhibition 32 Ambucetamide Antispasmodic 33 Amethocaine Local anaesthetic 34 Amfepramone Stimulant 35 Amfetaminil Stimulant 36 Amidephrine Vasoconstrictor 37 Amiloride Diuretic 1 Prohibited Substances List This is the Equine Prohibited Substances List that was voted in at the FEI General Assembly in November 2009 alongside the new Equine Anti-Doping and Controlled Medication Regulations(EADCMR). -

Kentucky Horse Racing Commission Withdrawal Guidelines Thoroughbred; Standardbred; Quarter Horse, Appaloosa, and Arabian KHRC 8-020-2 (11/2018)
Kentucky Horse Racing Commission Withdrawal Guidelines Thoroughbred; Standardbred; Quarter Horse, Appaloosa, and Arabian KHRC 8-020-2 (11/2018) General Notice Unless otherwise specified in these withdrawal guidelines or the applicable regulations and statutes, the following withdrawal guidelines are voluntary and advisory. The guidelines are recommendations based on current scientific knowledge that may change over time. A licensee may present evidence of full compliance with these guidelines to the Kentucky Horse Racing Commission (the “Commission” or “KHRC”) and the stewards as a mitigating factor to be used in determining violations and penalties. These withdrawal interval guidelines assume that administration of medications will be performed at doses that are not greater than the manufacturer’s maximum recommended dosage. Medications administered at dosages above manufacturer’s recommendations, in compounded formulations and/or in combination with other medications and/or administration inside the withdrawal interval may result in test sample concentrations above threshold concentrations that could lead to positive test results and the imposition of penalties. The time of administration of an orally administered substance, for the purposes of withdrawal interval, shall be considered to be the time of complete ingestion of the medication by the horse via eating or drinking. Brand names of medications, where applicable, are listed in parentheses or brackets following the generic name of a drug. In addition to the requirements contained in KRS Chapter 13A, the KHRC shall give notice of an amendment or addition to these withdrawal guidelines by posting the change on the KHRC website and at all Kentucky racetracks at least two weeks before the amendment or addition takes legal effect. -

Gc/Ms Assays for Abused Drugs in Body Fluids
GC/MS ASSAYS FOR ABUSED DRUGS IN BODY FLUIDS U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES • Public Health Service • Alchol, Drug Abuse, and Mental Health Administration GC/MS Assays for Abused Drugs in Body Fluids Rodger L. Foltz, Ph.D. Center for Human Toxicology University of Utah Salt Lake City, Utah 64112 Allison F. Fentiman, Jr., Ph.D. Ruth B. Foltz Battelle Columbus Laboratories Columbus, Ohio 43201 NIDA Research Monograph 32 August 1980 DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Alcohol, Drug Abuse, and Mental Health Administration National Institute on Drug Abuse Division of Research 5600 Fishers Lane Rockville, Maryland 20857 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 The NIDA Research Monograph series is prepared by the Division of Research of the National Institute on Drug Abuse. Its primary objective is to provide critical reviews of research problem areas and techniques, the content of state-of-the- art conferences, integrative research reviews and significant original research. Its dual publication emphasis is rapid and targeted dissemination to the scientific and professional community. Editorial Advisory Board Avram Goldstein, M.D. Addiction Research Foundation Palo Alto, California Jerome Jaffe, M.D. College of Physicians and Surgeons Columbia University, New York Reese T. Jones, M.D. Langley Porter Neuropsychiatric Institute University of California San Francisco, California William McGlothlin, Ph.D. Department of Psychology, UCLA Los Angeles, California Jack Mendelson, M.D. Alchol and Drug Abuse Research Center Harvard Medical School Mclean Hospital Belmont, Massachusetts Helen Nowlis, Ph.D. Office of Drug Education, DHHS Washington, D.C.